当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heme Binding to Porphobilinogen Deaminase from Vibrio cholerae Decelerates the Formation of 1-Hydroxymethylbilane
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1021/acschembio.7b00934 Takeshi Uchida 1, 2 , Takumi Funamizu 2 , Minghao Chen 3 , Yoshikazu Tanaka 4, 5 , Koichiro Ishimori 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1021/acschembio.7b00934 Takeshi Uchida 1, 2 , Takumi Funamizu 2 , Minghao Chen 3 , Yoshikazu Tanaka 4, 5 , Koichiro Ishimori 1, 2
Affiliation
|
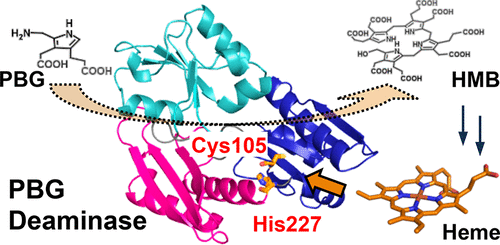
Porphobilinogen deaminase (PBGD) is an enzyme that catalyzes the formation of hydroxymethylbilane, a tetrapyrrole intermediate, during heme biosynthesis through the stepwise polymerization of four molecules of porphobilinogen. PBGD from Vibrio cholerae was expressed in Escherichia coli and characterized in this study. Unexpectedly, spectroscopic measurements revealed that PBGD bound one equivalent of heme with a dissociation constant of 0.33 ± 0.01 μM. The absorption and resonance Raman spectra suggested that heme is a mixture of the 5-coordinate and 6-coordinate hemes. Mutational studies indicated that the 5-coordinate heme possessed Cys105 as a heme axial ligand, and His227 was coordinated to form the 6-coordinate heme. Upon heme binding, the deamination activity decreased by approximately 15%. The crystal structure of PBGD revealed that His227 was located near Cys105, but the side chain of His227 did not point toward Cys105. The addition of the cyanide ion to heme–PBGD abolished the effect of heme binding on the enzymatic activity. Therefore, coordination of His227 to heme appeared to induce reorientation of the domains containing Cys105, leading to a decrease in the enzymatic activity. This is the first report indicating that the PBGD activity is controlled by heme, the final product of heme biosynthesis. This finding improves our understanding of the mechanism by which heme biosynthesis is regulated.
中文翻译:

血红素结合到霍乱弧菌的胆色素原脱氨酶上,会减慢1-羟甲基胆烷的形成。
猪胆色素原脱氨酶(PBGD)是一种酶,它通过逐步聚合四个分子胆色素原而催化血红素生物合成过程中四吡咯中间体羟甲基胆烷的形成。霍乱弧菌的PBGD在大肠杆菌中表达并在这项研究中具有特征。出乎意料的是,光谱测量结果表明PBGD结合了一当量的血红素,其解离常数为0.33±0.01μM。吸收和共振拉曼光谱表明血红素是5坐标和6坐标血红素的混合物。突变研究表明,5坐标血红素拥有Cys105作为血红素轴向配体,而His227被协调形成6坐标血红素。血红素结合后,脱氨活性降低了约15%。PBGD的晶体结构表明His227位于Cys105附近,但His227的侧链没有指向Cys105。在血红素-PBGD中添加氰化物离子消除了血红素结合对酶活性的影响。所以,His227与血红素的配位似乎诱导了含有Cys105的结构域的重新定向,从而导致了酶活性的降低。这是第一份表明PBGD活性受血红素(血红素生物合成的最终产物)控制的报告。这一发现增进了我们对血红素生物合成调控机制的理解。
更新日期:2018-01-23
中文翻译:

血红素结合到霍乱弧菌的胆色素原脱氨酶上,会减慢1-羟甲基胆烷的形成。
猪胆色素原脱氨酶(PBGD)是一种酶,它通过逐步聚合四个分子胆色素原而催化血红素生物合成过程中四吡咯中间体羟甲基胆烷的形成。霍乱弧菌的PBGD在大肠杆菌中表达并在这项研究中具有特征。出乎意料的是,光谱测量结果表明PBGD结合了一当量的血红素,其解离常数为0.33±0.01μM。吸收和共振拉曼光谱表明血红素是5坐标和6坐标血红素的混合物。突变研究表明,5坐标血红素拥有Cys105作为血红素轴向配体,而His227被协调形成6坐标血红素。血红素结合后,脱氨活性降低了约15%。PBGD的晶体结构表明His227位于Cys105附近,但His227的侧链没有指向Cys105。在血红素-PBGD中添加氰化物离子消除了血红素结合对酶活性的影响。所以,His227与血红素的配位似乎诱导了含有Cys105的结构域的重新定向,从而导致了酶活性的降低。这是第一份表明PBGD活性受血红素(血红素生物合成的最终产物)控制的报告。这一发现增进了我们对血红素生物合成调控机制的理解。































 京公网安备 11010802027423号
京公网安备 11010802027423号