Synthesis ( IF 2.2 ) Pub Date : 2018-01-22 , DOI: 10.1055/s-0036-1591887
Yoshihiko Yamamoto , Takashi Kurohara , Jiang Jiyue , Masatoshi Shibuya
|
Abstract
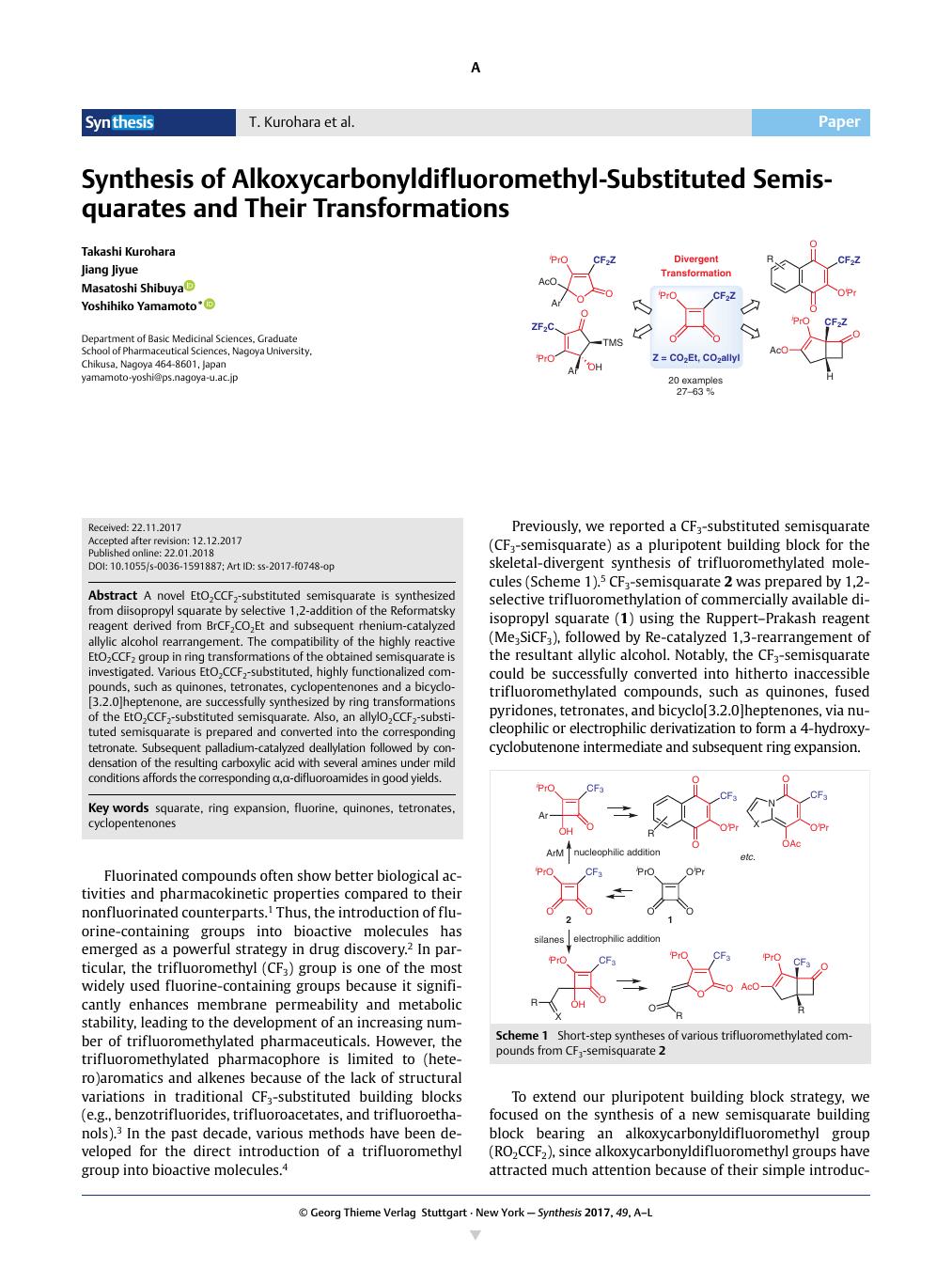
A novel EtO2CCF2-substituted semisquarate is synthesized from diisopropyl squarate by selective 1,2-addition of the Reformatsky reagent derived from BrCF2CO2Et and subsequent rhenium-catalyzed allylic alcohol rearrangement. The compatibility of the highly reactive EtO2CCF2 group in ring transformations of the obtained semisquarate is investigated. Various EtO2CCF2-substituted, highly functionalized compounds, such as quinones, tetronates, cyclopentenones and a bicyclo-[3.2.0]heptenone, are successfully synthesized by ring transformations of the EtO2CCF2-substituted semisquarate. Also, an allylO2CCF2-substituted semisquarate is prepared and converted into the corresponding tetronate. Subsequent palladium-catalyzed deallylation followed by condensation of the resulting carboxylic acid with several amines under mild conditions affords the corresponding α,α-difluoroamides in good yields.
A novel EtO2CCF2-substituted semisquarate is synthesized from diisopropyl squarate by selective 1,2-addition of the Reformatsky reagent derived from BrCF2CO2Et and subsequent rhenium-catalyzed allylic alcohol rearrangement. The compatibility of the highly reactive EtO2CCF2 group in ring transformations of the obtained semisquarate is investigated. Various EtO2CCF2-substituted, highly functionalized compounds, such as quinones, tetronates, cyclopentenones and a bicyclo-[3.2.0]heptenone, are successfully synthesized by ring transformations of the EtO2CCF2-substituted semisquarate. Also, an allylO2CCF2-substituted semisquarate is prepared and converted into the corresponding tetronate. Subsequent palladium-catalyzed deallylation followed by condensation of the resulting carboxylic acid with several amines under mild conditions affords the corresponding α,α-difluoroamides in good yields.
中文翻译:

烷氧羰基二氟甲基取代的半方形化合物的合成及其转化
摘要
一种新的EtO 2 CCF 2取代的半方酸酯是由二异丙基方酸酯通过选择性地1,2加成源自BrCF 2 CO 2 Et的Reformatsky试剂并随后进行-催化的烯丙基醇重排而合成的。研究了高反应性的EtO 2 CCF 2基团在所得半方形的环转化中的相容性。各种环氧乙烷2 CCF 2 -取代的,高功能的化合物,如醌,tetronates,环戊烯酮和二环- [3.2.0]庚烯酮,成功地由环氧乙烷的环转化合成2 CCF 2取代的半方形。同样,制备烯丙基O 2 CCF 2取代的半方酸酯,并将其转化为相应的tetronate。随后的钯催化的脱铝反应,然后在温和的条件下将所得的羧酸与几种胺进行缩合,可以以良好的收率得到相应的α,α-二氟酰胺。
一种新的EtO 2 CCF 2取代的半方酸酯是由二异丙基方酸酯通过选择性地1,2加成源自BrCF 2 CO 2 Et的Reformatsky试剂并随后进行-催化的烯丙基醇重排而合成的。研究了高反应性的EtO 2 CCF 2基团在所得半方形的环转化中的相容性。各种环氧乙烷2 CCF 2 -取代的,高功能的化合物,如醌,tetronates,环戊烯酮和二环- [3.2.0]庚烯酮,成功地由环氧乙烷的环转化合成2 CCF 2取代的半方形。同样,制备烯丙基O 2 CCF 2取代的半方酸酯,并将其转化为相应的tetronate。随后的钯催化的脱铝反应,然后在温和的条件下将所得的羧酸与几种胺进行缩合,可以以良好的收率得到相应的α,α-二氟酰胺。

































 京公网安备 11010802027423号
京公网安备 11010802027423号