当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isobaric Vapor–Liquid Equilibrium Data for Two Binary Systems n-Hexane + 1,2-Dimethoxyethane and Methylcyclopentane + 1,2-Dimethoxyethane at 101.3 kPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-01-22 00:00:00 , DOI: 10.1021/acs.jced.7b00802
Yinhai Sun 1 , Dianliang Fu 1 , Shoutao Ma 1 , Zhanhua Ma 1 , Lanyi Sun 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-01-22 00:00:00 , DOI: 10.1021/acs.jced.7b00802
Yinhai Sun 1 , Dianliang Fu 1 , Shoutao Ma 1 , Zhanhua Ma 1 , Lanyi Sun 1
Affiliation
|
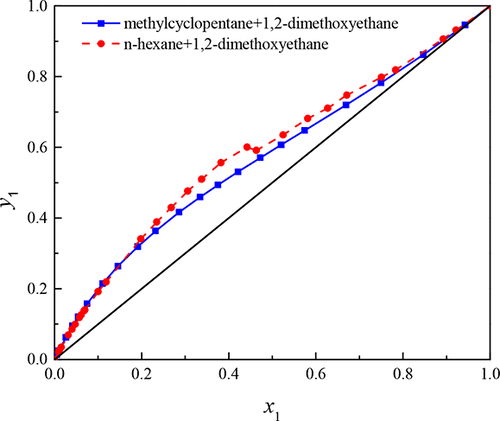
Isobaric vapor–liquid equilibrium (VLE) data for binary systems of n-hexane + 1,2-dimethoxyethane and methylcyclopentane + 1,2-dimethoxyethane were measured at 101.3 kPa with an Ellis vapor–liquid equilibrium still, and the thermodynamic consistency of the experimental data was checked and passed by the Herington method and Wisniak test. The VLE values were correlated with the nonrandom two-liquid, universal quasichemical and Wilson activity coefficient models, and the corresponding binary interaction parameters of the three models were obtained. The results correlated with three models have good agreement with the experimental data, indicating that the three models were all fitted well.
中文翻译:

等压汽液平衡数据二元系统Ñ己烷+ 1,2-二甲氧基乙烷和甲基环戊烷+ 1,2-二甲氧基乙烷在101.3kPa
等压汽-液平衡(VLE)为二进制系统中的数据Ñ己烷+ 1,2-二甲氧基乙烷和甲基环戊烷+ 1,2-二甲氧基乙烷中在101.3kPa测量与埃利斯气液平衡仍然,和的热力学一致性通过Herington方法和Wisniak检验检查并通过了实验数据。将VLE值与非随机两液,通用拟化学和Wilson活性系数模型相关联,并获得了三个模型的相应二元相互作用参数。与这三个模型相关的结果与实验数据吻合良好,表明这三个模型都拟合得很好。
更新日期:2018-01-22
中文翻译:

等压汽液平衡数据二元系统Ñ己烷+ 1,2-二甲氧基乙烷和甲基环戊烷+ 1,2-二甲氧基乙烷在101.3kPa
等压汽-液平衡(VLE)为二进制系统中的数据Ñ己烷+ 1,2-二甲氧基乙烷和甲基环戊烷+ 1,2-二甲氧基乙烷中在101.3kPa测量与埃利斯气液平衡仍然,和的热力学一致性通过Herington方法和Wisniak检验检查并通过了实验数据。将VLE值与非随机两液,通用拟化学和Wilson活性系数模型相关联,并获得了三个模型的相应二元相互作用参数。与这三个模型相关的结果与实验数据吻合良好,表明这三个模型都拟合得很好。

































 京公网安备 11010802027423号
京公网安备 11010802027423号