Nature Reviews Drug Discovery ( IF 122.7 ) Pub Date : 2018-01-19 , DOI: 10.1038/nrd.2017.244 Paul Morgan 1 , Dean G Brown 2 , Simon Lennard 3 , Mark J Anderton 1 , J Carl Barrett 4 , Ulf Eriksson 5 , Mark Fidock 6 , Bengt Hamrén 5 , Anthony Johnson 7 , Ruth E March 6 , James Matcham 7 , Jerome Mettetal 8 , David J Nicholls 9 , Stefan Platz 1 , Steve Rees 9 , Michael A Snowden 9 , Menelas N Pangalos 3
|
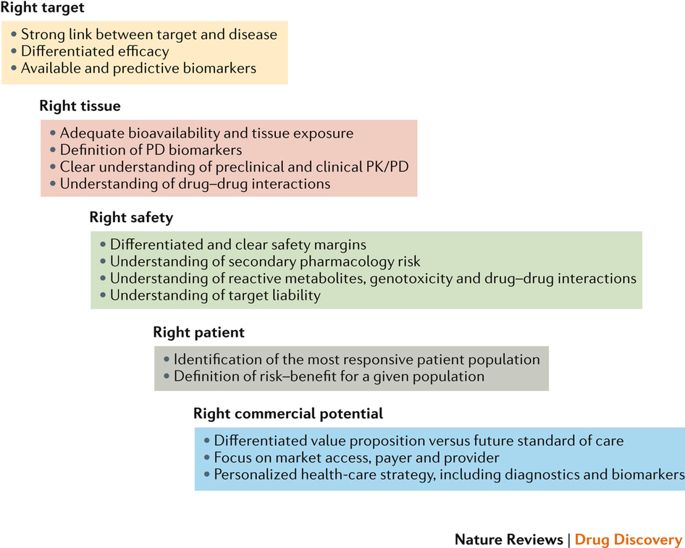
In 2011, AstraZeneca embarked on a major revision of its research and development (R&D) strategy with the aim of improving R&D productivity, which was below industry averages in 2005–2010. A cornerstone of the revised strategy was to focus decision-making on five technical determinants (the right target, right tissue, right safety, right patient and right commercial potential). In this article, we describe the progress made using this '5R framework' in the hope that our experience could be useful to other companies tackling R&D productivity issues. We focus on the evolution of our approach to target validation, hit and lead optimization, pharmacokinetic/pharmacodynamic modelling and drug safety testing, which have helped improve the quality of candidate drug nomination, as well as the development of the right culture, where 'truth seeking' is encouraged by more rigorous and quantitative decision-making. We also discuss where the approach has failed and the lessons learned. Overall, the continued evolution and application of the 5R framework are beginning to have an impact, with success rates from candidate drug nomination to phase III completion improving from 4% in 2005–2010 to 19% in 2012–2016.
中文翻译:

五维框架对阿斯利康的研发生产率的影响。
2011年,阿斯利康(AstraZeneca)开始对其研发(R&D)策略进行重大修订,目的是提高研发生产率,该水平低于2005-2010年的行业平均水平。修订后的战略的基础是将决策重点放在五个技术决定因素(正确的目标,正确的组织,正确的安全性,正确的患者和正确的商业潜力)上。在本文中,我们描述了使用“ 5R框架”取得的进展,希望我们的经验可以对其他解决研发生产力问题的公司有所帮助。我们专注于目标验证,命中和潜在顾客优化,药代动力学/药效学建模和药物安全性测试方法的演变,这些方法有助于提高候选药物提名的质量,并发展了正确的文化,更加严格和定量的决策鼓励“寻求真相”。我们还将讨论该方法的失败之处和经验教训。总体而言,5R框架的持续发展和应用开始产生影响,从候选药物提名到第三阶段完成的成功率从2005-2010年的4%提高到2012-2016年的19%。































 京公网安备 11010802027423号
京公网安备 11010802027423号