当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Far-Red Fluorescent Probe for Imaging of Vicinal Dithiol-Containing Proteins in Living Cells Based on a pKa Shift Mechanism
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-02-02 00:00:00 , DOI: 10.1021/acs.analchem.7b05429 Shengrui Zhang 1, 2 , Guojun Chen 1 , Yuanyuan Wang 1 , Qin Wang 1, 2 , Yaogang Zhong 3 , Xiao-Feng Yang 1 , Zheng Li 3 , Hua Li 4
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-02-02 00:00:00 , DOI: 10.1021/acs.analchem.7b05429 Shengrui Zhang 1, 2 , Guojun Chen 1 , Yuanyuan Wang 1 , Qin Wang 1, 2 , Yaogang Zhong 3 , Xiao-Feng Yang 1 , Zheng Li 3 , Hua Li 4
Affiliation
|
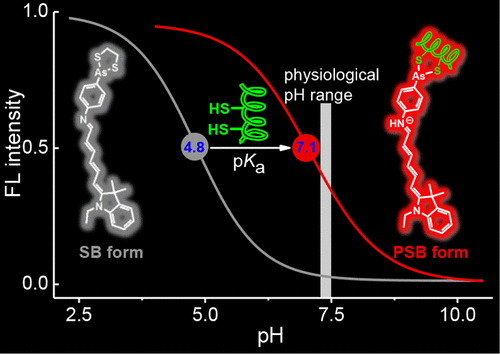
Vicinal dithiol-containing proteins (VDPs) play fundamental roles in intracellular redox homeostasis and are responsible for many diseases. In this work, we report a far-red fluorescence turn-on probe MCAs for VDPs exploiting the pKa shift of the imine functionality of the probe. MCAs is composed of a merocyanine Schiff base as the fluorescent reporter and a cyclic 1,3,2-dithiarsenolane as the specific ligand for VDPs. The imine pKa of MCAs is 4.8, and it exists predominantly in the Schiff base (SB) form at physiological pH. Due to the absence of a resonating positive charge, it absorbs at a relatively short wavelength and is essentially nonfluorescent. Upon selective binding to reduced bovine serum albumin (rBSA, selected as the model protein), MCAs was brought from aqueous media to the binding pockets of the protein, causing a large increase in pKa value of MCAs (pKa = 7.1). As a result, an increase in the protonated Schiff base (PSB) form of MCAs was observed at the physiological pH conditions, which in turn leads to a bathochromically shifted chromophore (λabs = 634 nm) and a significant increase in fluorescence intensity (λem = 657 nm) simultaneously. Furthermore, molecular dynamics simulations indicate that the salt bridges formed between the iminium in MCAs and the residues D72 and D517 in rBSA resist the dissociation of proton from the probe, thus inducing an increase of the pKa value. The proposed probe shows excellent sensitivity and specificity toward VDPs over other proteins and biologically relevant species and has been successfully applied for imaging of VDPs in living cells. We believe that the present pKa shift switching strategy may facilitate the development of new fluorescent probes that are useful for a wide range of applications.
中文翻译:

基于ap K a移位机理的活细胞中含邻二硫醇蛋白质的远红外荧光探针成像
含邻二硫醇的蛋白质(VDP)在细胞内氧化还原稳态中起着基本作用,并导致许多疾病。在这项工作中,我们报道了远红外荧光开启探头的MCA的VDPs利用该p ķ一个探头的亚胺功能转变。MCA由花色席夫碱作为荧光报告分子和环状1,3,2-二硫杂戊烯烷作为VDP的特定配体组成。亚胺p ķ一个的垄断管制是4.8,并且在生理pH下主要以席夫碱(SB)形式存在。由于不存在共振正电荷,因此它在相对短的波长处吸收并且基本上是无荧光的。在选择性结合还原的牛血清白蛋白(rBSA,被选作模型蛋白)后,MCA从水介质中带到该蛋白的结合口袋,导致MCAs的p K a值大大增加(p K a = 7.1)。 。结果,在生理pH条件下观察到MCA的质子化席夫碱(PSB)形式增加,继而导致红移的生色团(λabs= 634 nm)和同时显着增加的荧光强度(λem = 657 nm)。此外,分子动力学模拟表明,MCA中的亚胺与rBSA中的残基D72和D517之间形成的盐桥可阻止质子从探针上解离,从而导致p K a值增加。所提出的探针显示出对VDPs优于其他蛋白质和生物学相关物种的出色灵敏度和特异性,并已成功地用于活细胞中VDPs的成像。我们认为,目前的p ķ一个挡位切换策略可以方便的是为广泛的应用有用的新的荧光探针的发展。
更新日期:2018-02-02
中文翻译:

基于ap K a移位机理的活细胞中含邻二硫醇蛋白质的远红外荧光探针成像
含邻二硫醇的蛋白质(VDP)在细胞内氧化还原稳态中起着基本作用,并导致许多疾病。在这项工作中,我们报道了远红外荧光开启探头的MCA的VDPs利用该p ķ一个探头的亚胺功能转变。MCA由花色席夫碱作为荧光报告分子和环状1,3,2-二硫杂戊烯烷作为VDP的特定配体组成。亚胺p ķ一个的垄断管制是4.8,并且在生理pH下主要以席夫碱(SB)形式存在。由于不存在共振正电荷,因此它在相对短的波长处吸收并且基本上是无荧光的。在选择性结合还原的牛血清白蛋白(rBSA,被选作模型蛋白)后,MCA从水介质中带到该蛋白的结合口袋,导致MCAs的p K a值大大增加(p K a = 7.1)。 。结果,在生理pH条件下观察到MCA的质子化席夫碱(PSB)形式增加,继而导致红移的生色团(λabs= 634 nm)和同时显着增加的荧光强度(λem = 657 nm)。此外,分子动力学模拟表明,MCA中的亚胺与rBSA中的残基D72和D517之间形成的盐桥可阻止质子从探针上解离,从而导致p K a值增加。所提出的探针显示出对VDPs优于其他蛋白质和生物学相关物种的出色灵敏度和特异性,并已成功地用于活细胞中VDPs的成像。我们认为,目前的p ķ一个挡位切换策略可以方便的是为广泛的应用有用的新的荧光探针的发展。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号