当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Polysubstituted Quinolines from α-2-Aminoaryl Alcohols Via Nickel-Catalyzed Dehydrogenative Coupling
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-31 00:00:00 , DOI: 10.1021/acs.joc.7b03198
Sanju Das 1 , Debabrata Maiti 1 , Suman De Sarkar 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-31 00:00:00 , DOI: 10.1021/acs.joc.7b03198
Sanju Das 1 , Debabrata Maiti 1 , Suman De Sarkar 1
Affiliation
|
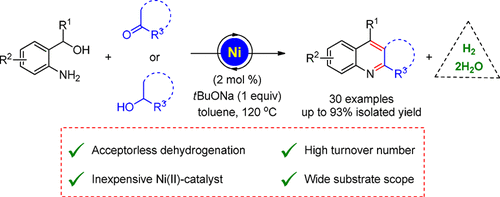
This study reports a nickel-catalyzed sustainable synthesis of polysubstituted quinolines from α-2-aminoaryl alcohols by a sequential dehydrogenation and condensation process that offers the advantages of a low catalyst loading and wide substrate scope. In contrast to earlier reported methods, this strategy allows the use of both primary as well as secondary α-2-aminoaryl alcohols in combination with either ketones or secondary alcohols for desired product formation. Using this methodology, 30 substituted quinoline derivatives were synthesized with up to 93% isolated yields.
中文翻译:

镍催化脱氢偶联反应由α-2-氨基芳基醇合成多取代喹啉
这项研究报告了通过顺序的脱氢和缩合工艺,由α-2-氨基芳基醇在镍催化下可持续合成多取代喹啉,具有低催化剂负载和广泛的底物范围的优点。与较早报道的方法相反,该策略允许同时使用伯和仲α-2-氨基芳基醇以及酮或仲醇,以形成所需的产物。使用这种方法,可以合成30种取代的喹啉衍生物,分离产率高达93%。
更新日期:2018-01-31
中文翻译:

镍催化脱氢偶联反应由α-2-氨基芳基醇合成多取代喹啉
这项研究报告了通过顺序的脱氢和缩合工艺,由α-2-氨基芳基醇在镍催化下可持续合成多取代喹啉,具有低催化剂负载和广泛的底物范围的优点。与较早报道的方法相反,该策略允许同时使用伯和仲α-2-氨基芳基醇以及酮或仲醇,以形成所需的产物。使用这种方法,可以合成30种取代的喹啉衍生物,分离产率高达93%。































 京公网安备 11010802027423号
京公网安备 11010802027423号