当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C?H Bond Activation by Early Transition Metal Carbide Cluster Anion MoC3−
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-10-22 , DOI: 10.1002/chem.201503060 Zi‐Yu Li , Lianrui Hu , Qing‐Yu Liu , Chuan‐Gang Ning , Hui Chen , Sheng‐Gui He , Jiannian Yao
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-10-22 , DOI: 10.1002/chem.201503060 Zi‐Yu Li , Lianrui Hu , Qing‐Yu Liu , Chuan‐Gang Ning , Hui Chen , Sheng‐Gui He , Jiannian Yao
|
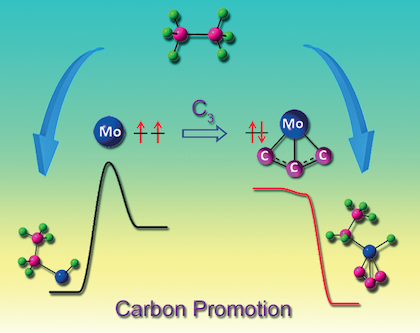
Although early transition metal (ETM) carbides can activate CH bonds in condensed‐phase systems, the electronic‐level mechanism is unclear. Atomic clusters are ideal model systems for understanding the mechanisms of bond activation. For the first time, CH activation of a simple alkane (ethane) by an ETM carbide cluster anion (MoC3−) under thermal‐collision conditions has been identified by using high‐resolution mass spectrometry, photoelectron imaging spectroscopy, and high‐level quantum chemical calculations. Dehydrogenation and ethene elimination were observed in the reaction of MoC3− with C2H6. The CH activation follows a mechanism of oxidative addition that is much more favorable in the carbon‐stabilized low‐spin ground electronic state than in the high‐spin excited state. The reaction efficiency between the MoC3− anion and C2H6 is low (0.23±0.05) %. A comparison between the anionic and a highly efficient cationic reaction system (Pt++C2H6) was made. It turned out that the potential‐energy surfaces for the entrance channels of the anionic and cationic reaction systems can be very different.
中文翻译:

C ?早期过渡金属碳化物簇阴离子MoC3-的H键活化
尽管早期过渡金属(ETM)碳化物可以在凝聚相系统中激活CH键,但电子能级机制尚不清楚。原子簇是理解键活化机理的理想模型系统。首次,C H A简单烷烃(乙烷)由ETM碳化物簇阴离子的活化(MOC 3 - )的热碰撞条件已被确定,通过使用高分辨率质谱,光电子摄像光谱下,和高级量子化学计算。脱氢和乙烯消除moc的反应中观察到3 -为C 2 ħ 6。的C H活化遵循一种氧化加成机理,在碳稳定的低自旋基态电子状态下,比在高自旋激发态下更有利。在交通部之间的反应效率3 -阴离子和C 2 H ^ 6为低(0.23±0.05)%。进行了阴离子和高效阳离子反应体系(Pt + + C 2 H 6)的比较。事实证明,阴离子和阳离子反应系统入口通道的势能表面可能非常不同。
更新日期:2015-10-22
中文翻译:

C ?早期过渡金属碳化物簇阴离子MoC3-的H键活化
尽管早期过渡金属(ETM)碳化物可以在凝聚相系统中激活CH键,但电子能级机制尚不清楚。原子簇是理解键活化机理的理想模型系统。首次,C H A简单烷烃(乙烷)由ETM碳化物簇阴离子的活化(MOC 3 - )的热碰撞条件已被确定,通过使用高分辨率质谱,光电子摄像光谱下,和高级量子化学计算。脱氢和乙烯消除moc的反应中观察到3 -为C 2 ħ 6。的C H活化遵循一种氧化加成机理,在碳稳定的低自旋基态电子状态下,比在高自旋激发态下更有利。在交通部之间的反应效率3 -阴离子和C 2 H ^ 6为低(0.23±0.05)%。进行了阴离子和高效阳离子反应体系(Pt + + C 2 H 6)的比较。事实证明,阴离子和阳离子反应系统入口通道的势能表面可能非常不同。

































 京公网安备 11010802027423号
京公网安备 11010802027423号