当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intensified Crystallization Processes for 1:1 Drug–Drug Cocrystals of Sulfathiazole–Theophylline, and Sulfathiazole–Sulfanilamide
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.cgd.7b01197 Kuan Lin Yeh,Tu Lee
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.cgd.7b01197 Kuan Lin Yeh,Tu Lee
|
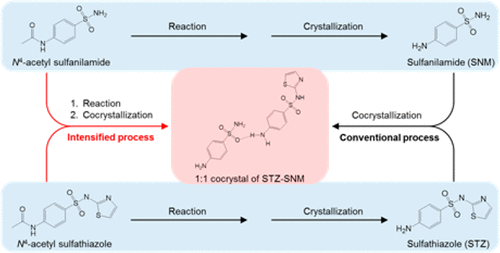
The chemical synthesis and crystallization steps were integrated successfully for directly producing a 1:1 cocrystal of sulfathiazole–theophylline and a 1:1 cocrystal of sulfathiazole–sulfanilamide. The benefits of this process intensification were the reduction of number of steps, and the amount of energy consumption and solvent used. In addition, the overall cocrystal yields by Intensified Method I were much higher than the ones by the conventional method. Intensified Method I also gave high-purity cocrystals of ≥99%. Sulfathiazole not forming cocrystals with sulfanilamide by Intensified Method I was dissolved in the mother liquor by taking advantage of the pH-dependent solubility of sulfathiazole. Cocrystals of both sulfathiazole–theophylline and sulfathiazole–sulfanilamide systems remained stable under conditions of 40 °C and 75% relative humidity for a month.
中文翻译:

磺胺噻唑-茶碱和磺胺噻唑-磺酰胺的1:1药物-药物共结晶的强化结晶过程
化学合成和结晶步骤已成功集成,可直接生产出硫代噻唑-茶碱的1:1共晶体和硫代噻唑-磺酰胺的1:1共晶体。强化工艺的好处是减少了步骤数量,减少了能耗和溶剂用量。另外,强化方法I的总共晶产率比常规方法高得多。强化方法I还得到了≥99%的高纯度共晶体。通过磺胺噻唑的pH依赖性溶解度,通过强化方法I与磺胺不形成共结晶的磺胺噻唑溶解在母液中。
更新日期:2018-01-16
中文翻译:

磺胺噻唑-茶碱和磺胺噻唑-磺酰胺的1:1药物-药物共结晶的强化结晶过程
化学合成和结晶步骤已成功集成,可直接生产出硫代噻唑-茶碱的1:1共晶体和硫代噻唑-磺酰胺的1:1共晶体。强化工艺的好处是减少了步骤数量,减少了能耗和溶剂用量。另外,强化方法I的总共晶产率比常规方法高得多。强化方法I还得到了≥99%的高纯度共晶体。通过磺胺噻唑的pH依赖性溶解度,通过强化方法I与磺胺不形成共结晶的磺胺噻唑溶解在母液中。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号