当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transglutaminase mediated PEGylation of nanobodies for targeted nano-drug delivery†
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1039/c7tb03132g Tiantian Wu 1, 2, 3, 4, 5 , Hai Huang 1, 2, 3, 4, 5 , Yaping Sheng 1, 2, 3, 4, 5 , Hongdong Shi 1, 2, 3, 4, 5 , Yuanzeng Min 6, 7, 8, 9 , Yangzhong Liu 1, 2, 3, 4, 5
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1039/c7tb03132g Tiantian Wu 1, 2, 3, 4, 5 , Hai Huang 1, 2, 3, 4, 5 , Yaping Sheng 1, 2, 3, 4, 5 , Hongdong Shi 1, 2, 3, 4, 5 , Yuanzeng Min 6, 7, 8, 9 , Yangzhong Liu 1, 2, 3, 4, 5
Affiliation
|
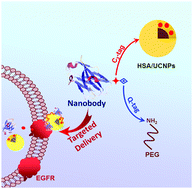
Targeted delivery of anticancer drugs that selectively accumulate in malignant cells could enhance drug efficacy and reduce side effects of conventional chemotherapy. In this work, we designed a single domain antibody (nanobody) based drug delivery system for targeted delivery of anticancer drugs. An anti-EGFR nanobody (Nb) was constructed with a C3-tag and a Q-tag for site specific modifications under physiological conditions. The site specific PEGylation of the nanobody was achieved via a transglutaminase catalyzed reaction through the coupling of the Q-tag with PEG-NH2. As a proof of concept, the PEGylated nanobody was tethered to HSA coated upconversion nanoparticles (UCNPs) through the C3-tag, and an anticancer drug, doxorubicin (DOX), was loaded. Results showed that the Nb-conjugated drug delivery system exhibits superior specificity to the EGFR positive tumor cells. The drug delivery system is highly accumulated in the EGFR positive tumor cells (A431), whereas there was no detectable accumulation in the EGFR negative cells (MCF-7). Consequently, the drug loaded particles demonstrated significantly higher anti-proliferation to A431 cells than to MCF-7 cells. This work provides an effective approach for site-specific modification of nanobodies for the construction of targeted drug delivery systems.
中文翻译:

转谷氨酰胺酶介导的纳米抗体的聚乙二醇化,可用于靶向纳米药物的递送†
有针对性地输送选择性积聚在恶性细胞中的抗癌药物可以增强药物疗效,并减少常规化学疗法的副作用。在这项工作中,我们设计了基于单域抗体(纳米抗体)的药物递送系统,用于靶向递送抗癌药物。构建具有C 3标签和Q标签的抗EGFR纳米抗体(Nb),以在生理条件下进行位点特异性修饰。通过Q-标签与PEG-NH 2的偶联,通过转谷氨酰胺酶催化的反应实现了纳米抗体的位点特异性PEG化。作为概念的证明,聚乙二醇化的纳米抗体通过C 3束缚到HSA涂层的上转换纳米颗粒(UCNP)标记,并加载了抗癌药阿霉素(DOX)。结果表明,结合Nb的药物递送系统对EGFR阳性肿瘤细胞表现出更高的特异性。药物输送系统在EGFR阳性肿瘤细胞(A431)中高度积累,而在EGFR阴性细胞(MCF-7)中没有可检测到的积累。因此,载药颗粒显示出对A431细胞的抗增殖能力明显高于对MCF-7细胞的抗增殖能力。这项工作提供了一种针对特定位置修饰纳米抗体的有效方法,用于构建靶向药物递送系统。
更新日期:2018-01-17
中文翻译:

转谷氨酰胺酶介导的纳米抗体的聚乙二醇化,可用于靶向纳米药物的递送†
有针对性地输送选择性积聚在恶性细胞中的抗癌药物可以增强药物疗效,并减少常规化学疗法的副作用。在这项工作中,我们设计了基于单域抗体(纳米抗体)的药物递送系统,用于靶向递送抗癌药物。构建具有C 3标签和Q标签的抗EGFR纳米抗体(Nb),以在生理条件下进行位点特异性修饰。通过Q-标签与PEG-NH 2的偶联,通过转谷氨酰胺酶催化的反应实现了纳米抗体的位点特异性PEG化。作为概念的证明,聚乙二醇化的纳米抗体通过C 3束缚到HSA涂层的上转换纳米颗粒(UCNP)标记,并加载了抗癌药阿霉素(DOX)。结果表明,结合Nb的药物递送系统对EGFR阳性肿瘤细胞表现出更高的特异性。药物输送系统在EGFR阳性肿瘤细胞(A431)中高度积累,而在EGFR阴性细胞(MCF-7)中没有可检测到的积累。因此,载药颗粒显示出对A431细胞的抗增殖能力明显高于对MCF-7细胞的抗增殖能力。这项工作提供了一种针对特定位置修饰纳米抗体的有效方法,用于构建靶向药物递送系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号