Current Opinion in Microbiology ( IF 5.9 ) Pub Date : 2018-01-12 , DOI: 10.1016/j.mib.2017.12.010 Filipa L Sousa , Martina Preiner , William F Martin
|
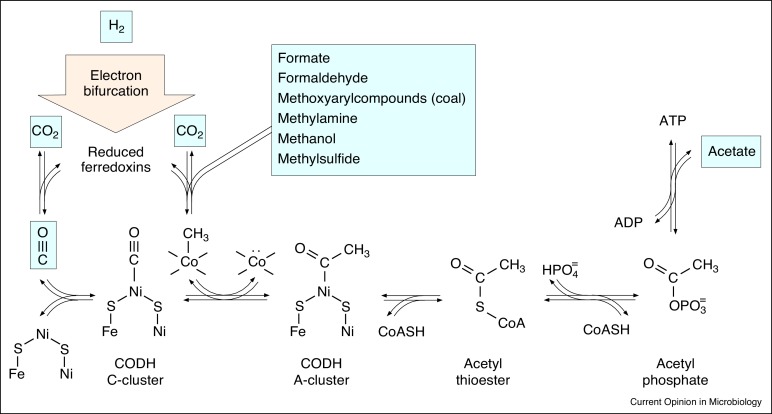
Molecular hydrogen is an ancient source of energy and electrons. Anaerobic autotrophs that harness the H2/CO2 redox couple harbour ancient biochemical traits that trace back to the universal common ancestor. Aspects of their physiology, including the abundance of transition metals, radical reaction mechanisms, and their main exergonic bioenergetic reactions, forge links between ancient microbes and geochemical reactions at hydrothermal vents. The midpoint potential of H2 however requires anaerobes that reduce CO2 with H2 to use flavin based electron bifurcation — a mechanism to conserve energy as low potential reduced ferredoxins via soluble proteins — for CO2 fixation. This presents a paradox. At the onset of biochemical evolution, before there were proteins, how was CO2 reduced using H2? FeS minerals alone are probably not the solution, because biological CO2 reduction is a two electron reaction. Physiology can provide clues. Some acetogens and some methanogens can grow using native iron (Fe0) instead of H2 as the electron donor. In the laboratory, Fe0 efficiently reduces CO2 to acetate and methanol. Hydrothermal vents harbour awaruite, Ni3Fe, a natural compound of native metals. Native metals might have been the precursors of electron bifurcation in biochemical evolution.
中文翻译:

早期生物化学演化过程中的天然金属,电子分叉和CO 2还原
氢分子是古老的能量和电子来源。利用H 2 / CO 2氧化还原对的厌氧自养生物具有古老的生化特征,可追溯到普遍的祖先。它们的生理学方面,包括丰富的过渡金属,自由基反应机理及其主要的能谱生物能反应,在热液喷口处形成了古代微生物与地球化学反应之间的联系。但是,H 2的中点电势需要使用H 2来还原CO 2的厌氧菌,以黄素为基础的电子分叉—一种作为能量的机制,通过可溶性蛋白将铁氧还蛋白作为低电势还原为CO 2。固定。这提出了一个悖论。在生化进化的开始,之前有蛋白质,是如何CO 2使用H还原2?单独的FeS矿物质可能不是解决方案,因为生物CO 2还原是两个电子反应。生理学可以提供线索。使用天然铁(Fe 0)代替H 2作为电子供体,可以生长一些产乙酸原和一些产甲烷菌。在实验室中,Fe 0将CO 2有效还原为乙酸盐和甲醇。热液喷口含有极光辉石,Ni 3 Fe,一种天然的天然金属化合物。天然金属可能是生化进化中电子分叉的前体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号