当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extending Half Life of H-Ferritin Nanoparticle by Fusing Albumin Binding Domain for Doxorubicin Encapsulation
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.biomac.7b01545 Chunyue Wang 1, 2 , Chun Zhang 1, 2 , Zenglan Li 2 , Shuang Yin 1, 2, 3 , Qi Wang 2 , Fangxia Guo 2 , Yao Zhang 2 , Rong Yu 1 , Yongdong Liu 2 , Zhiguo Su 2
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.biomac.7b01545 Chunyue Wang 1, 2 , Chun Zhang 1, 2 , Zenglan Li 2 , Shuang Yin 1, 2, 3 , Qi Wang 2 , Fangxia Guo 2 , Yao Zhang 2 , Rong Yu 1 , Yongdong Liu 2 , Zhiguo Su 2
Affiliation
|
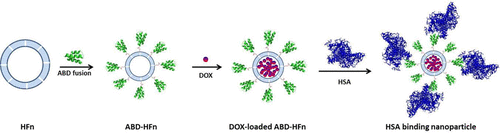
Nanoparticles based on the heavy chain of the human ferritin (HFn) are arousing growing interest in the field of drug delivery due to their exceptional characteristics. However, the unsatisfied plasma half life of HFn substantially limits its application as a delivery platform for antitumor agents. Herein we fused an albumin binding domain (ABD) variant that basically derives from the streptococcal protein G and possesses a long-acting characteristic in serum albumin to the N-terminus of the HFn for the aim of half-life extension. This ABD–HFn construct was highly expressed and fully self-assembled into symmetrical and spherical structure in E. coli bacteria. The purified ABD–HFn showed a similar particle size with wild-type HFn and also exhibited an extremely high binding affinity with human serum albumin. To evaluate the therapeutic potential of this ABD–HFn construct in terms of half-life extension, we encapsulated a model antitumor agent doxorubicin (DOX) into the ABD–HFn. Significantly outstanding loading efficacy of above 60 molecules doxorubicin for each ABD–HFn cage was achieved. The doxorubicin-loaded ABD–HFn nanoparticle was characterized and further compared with the recombinant HFn counterpart. The ABD–HFn/DOX nanoparticle showed dramatically improved stability and comparable cell uptake rate when compared with HFn/DOX counterpart. Pharmacokinetics study in Sprague–Dawley rats showed that ABD–HFn/DOX nanoparticle possessed significantly prolonged plasma half life of ∼17.2 h, exhibiting nearly 19 times longer than that of free doxorubicin and 12 times for HFn/DOX. These optimal results indicated that fusion with ABD will be a promising strategy to extend the half life for protein-based nanoparticles.
中文翻译:

通过融合白蛋白结合域的阿霉素封装来延长H-铁蛋白纳米颗粒的半衰期
基于人铁蛋白(HFn)重链的纳米颗粒因其卓越的特性而引起了人们对药物输送领域的日益增长的兴趣。但是,HFn的血浆半衰期不能令人满意,这极大地限制了其作为抗肿瘤药输送平台的应用。在本文中,我们融合了一个白蛋白结合域(ABD)变体,该变体基本上来自链球菌蛋白G,并在血清白蛋白中具有长效特性,从而达到延长半衰期的目的。这种ABD–HFn构建体在大肠杆菌中被高度表达并完全自组装成对称和球形结构细菌。纯化的ABD–HFn的粒径与野生型HFn相似,并且与人血清白蛋白的结合亲和力极高。为了评估半衰期延长这一ABD-HFn构建体的治疗潜力,我们将模型抗肿瘤药阿霉素(DOX)封装到ABD-HFn中。对于每个ABD-HFn笼子,超过60个分子的阿霉素具有显着优异的负载功效。载有阿霉素的ABD–HFn纳米颗粒的特征在于,与重组HFn对应物进行了进一步比较。与HFn / DOX相比,ABD–HFn / DOX纳米颗粒具有显着改善的稳定性和相当的细胞摄取率。在Sprague–Dawley大鼠中进行的药代动力学研究表明,ABD–HFn / DOX纳米颗粒的血浆半衰期显着延长,约为17.2小时,展示的时间比游离阿霉素长19倍,是HFn / DOX的12倍。这些最佳结果表明,与ABD融合将是延长基于蛋白质的纳米粒子半衰期的有前途的策略。
更新日期:2018-01-12
中文翻译:

通过融合白蛋白结合域的阿霉素封装来延长H-铁蛋白纳米颗粒的半衰期
基于人铁蛋白(HFn)重链的纳米颗粒因其卓越的特性而引起了人们对药物输送领域的日益增长的兴趣。但是,HFn的血浆半衰期不能令人满意,这极大地限制了其作为抗肿瘤药输送平台的应用。在本文中,我们融合了一个白蛋白结合域(ABD)变体,该变体基本上来自链球菌蛋白G,并在血清白蛋白中具有长效特性,从而达到延长半衰期的目的。这种ABD–HFn构建体在大肠杆菌中被高度表达并完全自组装成对称和球形结构细菌。纯化的ABD–HFn的粒径与野生型HFn相似,并且与人血清白蛋白的结合亲和力极高。为了评估半衰期延长这一ABD-HFn构建体的治疗潜力,我们将模型抗肿瘤药阿霉素(DOX)封装到ABD-HFn中。对于每个ABD-HFn笼子,超过60个分子的阿霉素具有显着优异的负载功效。载有阿霉素的ABD–HFn纳米颗粒的特征在于,与重组HFn对应物进行了进一步比较。与HFn / DOX相比,ABD–HFn / DOX纳米颗粒具有显着改善的稳定性和相当的细胞摄取率。在Sprague–Dawley大鼠中进行的药代动力学研究表明,ABD–HFn / DOX纳米颗粒的血浆半衰期显着延长,约为17.2小时,展示的时间比游离阿霉素长19倍,是HFn / DOX的12倍。这些最佳结果表明,与ABD融合将是延长基于蛋白质的纳米粒子半衰期的有前途的策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号