当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilizing Leaf and Branch Compost Cutinase (LCC) with Glycosylation: Mechanism and Effect on PET Hydrolysis
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-30 00:00:00 , DOI: 10.1021/acs.biochem.7b01189 Abhijit N. Shirke 1, 2 , Christine White 3 , Jacob A. Englaender 4 , Allison Zwarycz 4 , Glenn L. Butterfoss 5 , Robert J. Linhardt 1, 2, 3, 4 , Richard A. Gross 1, 2, 3
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-30 00:00:00 , DOI: 10.1021/acs.biochem.7b01189 Abhijit N. Shirke 1, 2 , Christine White 3 , Jacob A. Englaender 4 , Allison Zwarycz 4 , Glenn L. Butterfoss 5 , Robert J. Linhardt 1, 2, 3, 4 , Richard A. Gross 1, 2, 3
Affiliation
|
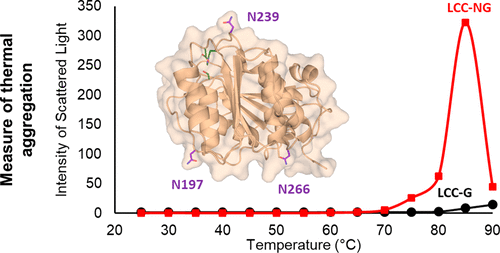
Cutinases are polyester hydrolases that show a remarkable capability to hydrolyze polyethylene terephthalate (PET) to its monomeric units. This revelation has stimulated research aimed at developing sustainable and green cutinase-catalyzed PET recycling methods. Leaf and branch compost cutinase (LCC) is particularly suited toward these ends given its relatively high PET hydrolysis activity and thermostability. Any practical enzymatic PET recycling application will require that the protein have kinetic stability at or above the PET glass transition temperature (Tg, i.e., 70 °C). This paper elucidates the thermodynamics and kinetics of LCC conformational and colloidal stability. Aggregation emerged as a major contributor that reduces LCC kinetic stability. In its native state, LCC is prone to aggregation owing to electrostatic interactions. Further, with increasing temperature, perturbation of LCC’s tertiary structure and corresponding exposure of hydrophobic domains leads to rapid aggregation. Glycosylation was employed in an attempt to impede LCC aggregation. Owing to the presence of three putative N-glycosylation sites, expression of native LCC in Pichia pastoris resulted in the production of glycosylated LCC (LCC-G). LCC-G showed improved stability to native state aggregation while increasing the temperature for thermal induced aggregation by 10 °C. Furthermore, stabilization against thermal aggregation resulted in improved catalytic PET hydrolysis both at its optimum temperature and concentration.
中文翻译:

糖基化稳定叶片和树枝堆肥角质酶(LCC):机理和对PET水解的影响
角质酶是聚酯水解酶,其显示出显着的将聚对苯二甲酸乙二醇酯(PET)水解成其单体单元的能力。这一发现激发了旨在开发可持续和绿色角质酶催化的PET回收方法的研究。叶片和树枝堆肥角质酶(LCC)特别适合这些目的,因为它具有较高的PET水解活性和热稳定性。任何实际的酶法PET回收应用都需要蛋白质在PET玻璃化转变温度(T g或更高)下具有动力学稳定性,即70°C)。本文阐明了LCC构象和胶体稳定性的热力学和动力学。聚集成为降低LCC动力学稳定性的主要因素。在其原始状态下,由于静电相互作用,LCC易于聚集。此外,随着温度升高,LCC的三级结构的扰动和疏水域的相应暴露导致快速聚集。使用糖基化试图阻止LCC聚集。由于存在三个推定的N-糖基化位点,天然LCC在毕赤酵母中的表达导致糖基化LCC(LCC-G)的产生。LCC-G表现出对原始状态聚集的改善的稳定性,同时将热诱导聚集的温度提高了10°C。此外,针对热聚集的稳定化在其最佳温度和浓度下均改善了催化PET水解。
更新日期:2018-01-30
中文翻译:

糖基化稳定叶片和树枝堆肥角质酶(LCC):机理和对PET水解的影响
角质酶是聚酯水解酶,其显示出显着的将聚对苯二甲酸乙二醇酯(PET)水解成其单体单元的能力。这一发现激发了旨在开发可持续和绿色角质酶催化的PET回收方法的研究。叶片和树枝堆肥角质酶(LCC)特别适合这些目的,因为它具有较高的PET水解活性和热稳定性。任何实际的酶法PET回收应用都需要蛋白质在PET玻璃化转变温度(T g或更高)下具有动力学稳定性,即70°C)。本文阐明了LCC构象和胶体稳定性的热力学和动力学。聚集成为降低LCC动力学稳定性的主要因素。在其原始状态下,由于静电相互作用,LCC易于聚集。此外,随着温度升高,LCC的三级结构的扰动和疏水域的相应暴露导致快速聚集。使用糖基化试图阻止LCC聚集。由于存在三个推定的N-糖基化位点,天然LCC在毕赤酵母中的表达导致糖基化LCC(LCC-G)的产生。LCC-G表现出对原始状态聚集的改善的稳定性,同时将热诱导聚集的温度提高了10°C。此外,针对热聚集的稳定化在其最佳温度和浓度下均改善了催化PET水解。































 京公网安备 11010802027423号
京公网安备 11010802027423号