Synthesis ( IF 2.2 ) Pub Date : 2018-01-11 , DOI: 10.1055/s-0036-1591879 Danny Müller 1 , Marco Seifried 1 , Christian Knoll 1 , Jan Welch 2 , Peter Weinberger 1
|
Abstract
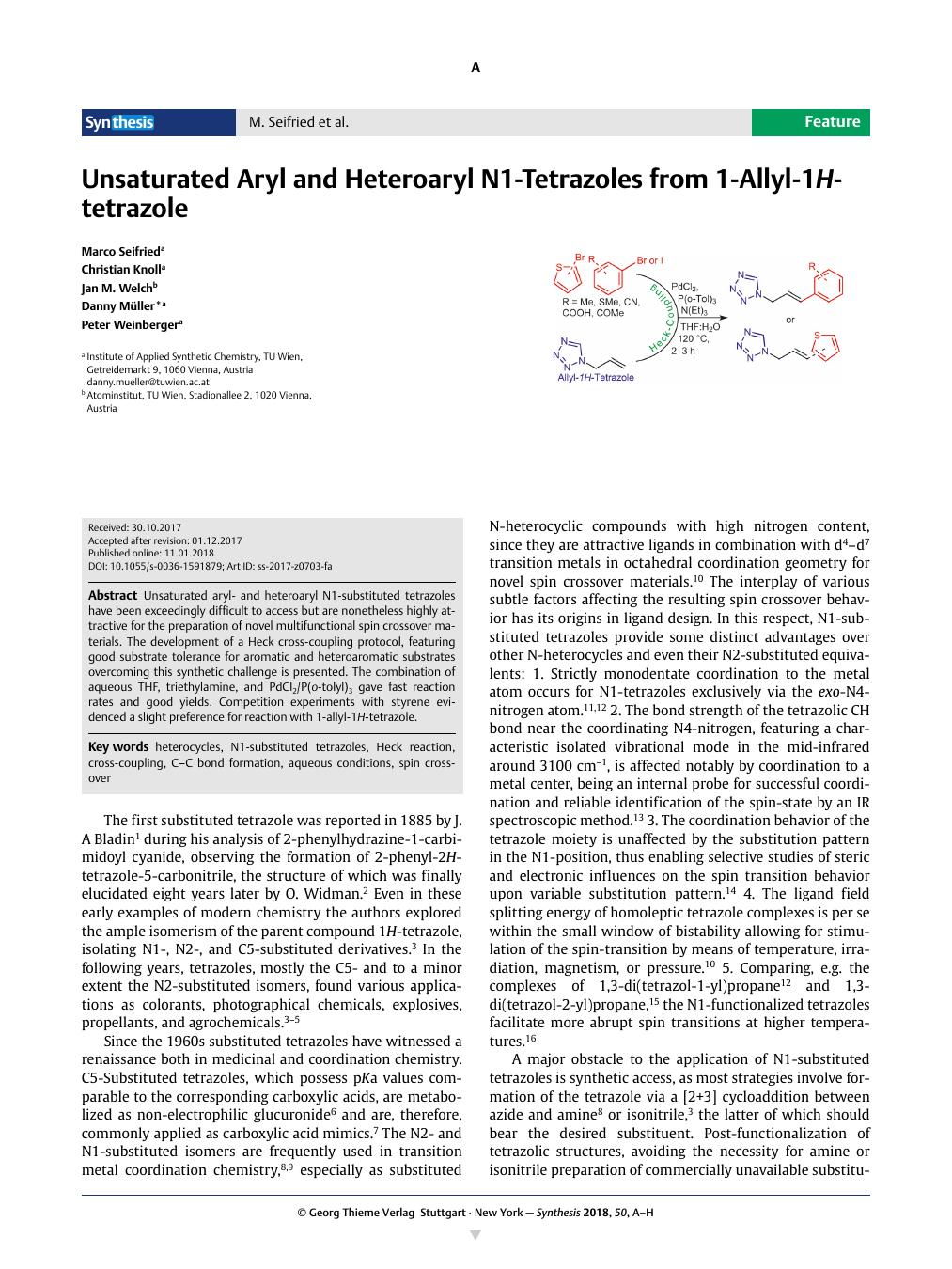
Unsaturated aryl- and heteroaryl N1-substituted tetrazoles have been exceedingly difficult to access but are nonetheless highly attractive for the preparation of novel multifunctional spin crossover materials. The development of a Heck cross-coupling protocol, featuring good substrate tolerance for aromatic and heteroaromatic substrates overcoming this synthetic challenge is presented. The combination of aqueous THF, triethylamine, and PdCl2/P(o-tolyl)3 gave fast reaction rates and good yields. Competition experiments with styrene evidenced a slight preference for reaction with 1-allyl-1H-tetrazole.
Unsaturated aryl- and heteroaryl N1-substituted tetrazoles have been exceedingly difficult to access but are nonetheless highly attractive for the preparation of novel multifunctional spin crossover materials. The development of a Heck cross-coupling protocol, featuring good substrate tolerance for aromatic and heteroaromatic substrates overcoming this synthetic challenge is presented. The combination of aqueous THF, triethylamine, and PdCl2/P(o-tolyl)3 gave fast reaction rates and good yields. Competition experiments with styrene evidenced a slight preference for reaction with 1-allyl-1H-tetrazole.
中文翻译:

1-烯丙基-1H-四唑的不饱和芳基和杂芳基N1-四唑
摘要
不饱和的芳基和杂芳基的N 1-取代的四唑已经极难接近,但是对于制备新型的多功能自旋交联材料具有很高的吸引力。提出了Heck交叉偶联方案的开发,该方案具有克服此合成难题的芳香族和杂芳香族底物良好的底物耐受性。THF,三乙胺和PdCl 2 / P(邻甲苯基)3水溶液的组合提供了快速的反应速率和良好的产率。与苯乙烯的竞争实验表明,偏爱与1-allyl-1 H-四唑反应。
不饱和的芳基和杂芳基的N 1-取代的四唑已经极难接近,但是对于制备新型的多功能自旋交联材料具有很高的吸引力。提出了Heck交叉偶联方案的开发,该方案具有克服此合成难题的芳香族和杂芳香族底物良好的底物耐受性。THF,三乙胺和PdCl 2 / P(邻甲苯基)3水溶液的组合提供了快速的反应速率和良好的产率。与苯乙烯的竞争实验表明,偏爱与1-allyl-1 H-四唑反应。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号