当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can an Alcohol Act As an Acid/Base Catalyst in Water Solution? An Experimental and Theoretical Study of Imidazole Catalysis of the Aqueous Morita–Baylis–Hillman Reaction
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1021/acscatal.7b04053 Lluís Raich 1, 2 , Hugo Santos 1, 3 , Juliana C. Gomes 3 , Manoel T. Rodrigues 3 , Renan Galaverna 4 , Marcos N. Eberlin 4 , Fernando Coelho 3 , Carme Rovira 1, 2, 5 , Albert Moyano 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1021/acscatal.7b04053 Lluís Raich 1, 2 , Hugo Santos 1, 3 , Juliana C. Gomes 3 , Manoel T. Rodrigues 3 , Renan Galaverna 4 , Marcos N. Eberlin 4 , Fernando Coelho 3 , Carme Rovira 1, 2, 5 , Albert Moyano 1
Affiliation
|
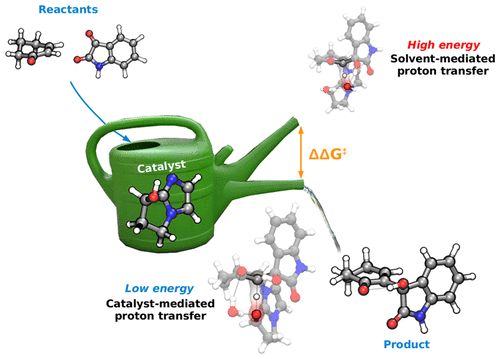
The formation of carbon–carbon sigma bonds by the organocatalyzed Morita–Baylis–Hillman (MBH) reaction constitutes a convenient method for the synthesis of valuable, highly functionalized molecules. Its large-scale implementation is however hampered both by its poor performance with substrates such as α,β-unsaturated ketones and by the reduction of the nucleophilicity of the catalyst when using water as a solvent. Recent work from our laboratories has shown that a bicyclic imidazolyl alcohol (BIA) overcomes these limitations and is a much more efficient catalyst than imidazole for the aqueous MBH reactions of cyclic enones. The role of the hydroxyl group in the former catalyst is not easy to understand, however, since these reactions take place in water solution. We have studied the mechanism of the aqueous MBH reaction between 2-cyclohexenone and isatin, catalyzed either by imidazole or by the BIA catalyst, using a combined experimental and computational approach. The data allowed us to propose mechanistic free-energy profiles for the two catalysts. An intramolecular proton transfer step, facilitated by the hydroxyl group of the catalyst even if the reaction takes place in water, accounts for the higher catalytic efficiency of BIA in comparison to imidazole, which requires assistance by an external base (either hydroxide ion or another imidazole molecule) for this catalytic step. The computed activation energies are in good agreement with the experimentally observed trends in reaction rates. The crucial role of the BIA hydroxyl has been confirmed by NMR study of the reaction kinetics, and in situ ESI-MS/MS monitoring experiments have detected and characterized all the relevant reaction intermediates, validating the computational model. This study provides clear evidence for the intramolecular participation of a bifunctional catalyst in the proton transfer step of an MBH reaction. The fact that the introduction of a suitable functional group favors the intramolecular proton transfer over solvent-mediated pathways, just in the spirit of enzymatic catalysis, provides a basis for the rational design of future efficient catalysts for aqueous reactions.
中文翻译:

醇可以在水溶液中充当酸/碱催化剂吗?咪唑催化Morita-Baylis-Hillman反应的实验和理论研究
有机催化的森田-贝利斯-希尔曼(MBH)反应形成的碳-碳sigma键构成了合成有价值的,高度官能化的分子的便捷方法。然而,由于其对诸如α,β-不饱和酮之类的底物的不良性能以及当使用水作为溶剂时催化剂的亲核性降低,阻碍了其大规模实施。我们实验室的最新工作表明,双环咪唑醇(BIA)克服了这些限制,对于环烯酮的MBH水溶液反应,它比咪唑更有效。然而,由于这些反应在水溶液中进行,因此在前一种催化剂中羟基的作用并不容易理解。我们使用组合的实验和计算方法,研究了咪唑或BIA催化剂催化2-环己烯酮和靛红之间MBH水溶液反应的机理。数据允许我们提出两种催化剂的机械自由能曲线。与咪唑相比,即使在水中发生反应,催化剂的羟基也可促进分子内质子转移步骤,因为BIA与咪唑相比需要更高的催化效率,咪唑需要外部碱(氢氧根离子或另一种咪唑)的辅助分子)用于该催化步骤。计算出的活化能与实验观察到的反应速率趋势非常吻合。核磁共振研究反应动力学证实了BIA羟基的关键作用,现场ESI-MS / MS监测实验已经检测并表征了所有相关的反应中间体,从而验证了计算模型。这项研究为双功能催化剂在MBH反应的质子转移步骤中的分子内参与提供了明确的证据。正好在酶催化的精神上,引入合适的官能团比溶剂介导的途径更有利于分子内质子转移,这一事实为合理设计用于水反应的未来有效催化剂提供了基础。
更新日期:2018-01-29
中文翻译:

醇可以在水溶液中充当酸/碱催化剂吗?咪唑催化Morita-Baylis-Hillman反应的实验和理论研究
有机催化的森田-贝利斯-希尔曼(MBH)反应形成的碳-碳sigma键构成了合成有价值的,高度官能化的分子的便捷方法。然而,由于其对诸如α,β-不饱和酮之类的底物的不良性能以及当使用水作为溶剂时催化剂的亲核性降低,阻碍了其大规模实施。我们实验室的最新工作表明,双环咪唑醇(BIA)克服了这些限制,对于环烯酮的MBH水溶液反应,它比咪唑更有效。然而,由于这些反应在水溶液中进行,因此在前一种催化剂中羟基的作用并不容易理解。我们使用组合的实验和计算方法,研究了咪唑或BIA催化剂催化2-环己烯酮和靛红之间MBH水溶液反应的机理。数据允许我们提出两种催化剂的机械自由能曲线。与咪唑相比,即使在水中发生反应,催化剂的羟基也可促进分子内质子转移步骤,因为BIA与咪唑相比需要更高的催化效率,咪唑需要外部碱(氢氧根离子或另一种咪唑)的辅助分子)用于该催化步骤。计算出的活化能与实验观察到的反应速率趋势非常吻合。核磁共振研究反应动力学证实了BIA羟基的关键作用,现场ESI-MS / MS监测实验已经检测并表征了所有相关的反应中间体,从而验证了计算模型。这项研究为双功能催化剂在MBH反应的质子转移步骤中的分子内参与提供了明确的证据。正好在酶催化的精神上,引入合适的官能团比溶剂介导的途径更有利于分子内质子转移,这一事实为合理设计用于水反应的未来有效催化剂提供了基础。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号