当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocopper-Triggered Cyclisation of Conjugated Diene-ynes: Diastereo- and Enantioselective Synthesis of Indenes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-10-20 02:30:07 , DOI: 10.1002/adsc.201500556 Tanzeel Arif , Cyril Borie , Aura Tintaru , Jean-Valère Naubron , Nicolas Vanthuyne , Michèle P. Bertrand , Malek Nechab
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-10-20 02:30:07 , DOI: 10.1002/adsc.201500556 Tanzeel Arif , Cyril Borie , Aura Tintaru , Jean-Valère Naubron , Nicolas Vanthuyne , Michèle P. Bertrand , Malek Nechab
|
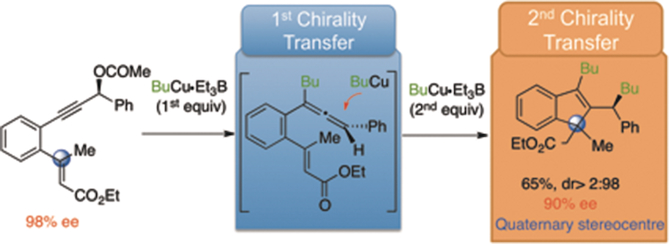
Organocopper reagents react with readily available chiral conjugated diene-ynes to give indene derivatives bearing two stereogenic centres. The investigation of this original reaction in optically pure series demonstrates that a double transfer of chirality is operating. A stereocontrolled cascade involving SN2′ followed by carbocupration and conjugate addition reactions accounts for the total recovery of the initial chirality. The scope and limitations of the reaction were investigated. The high diastereofacial discrimination in the cyclisation step allowed the construction of the quaternary stereocentre with excellent dr and ee, with the opposite configuration depending on the E- or Z-configuration of the alkene in the starting material. Post-functionalisation of indenes allowed the synthesis of indanyl derivatives containing four contiguous stereocentres.
中文翻译:

共轭二烯-炔的有机铜引发环化:茚的非对映和对映选择性合成
有机铜试剂与容易获得的手性共轭二烯-炔反应,得到带有两个立体异构中心的茚衍生物。在光学纯系列中对该原始反应的研究表明,手性的双重转移正在起作用。涉及S N 2'的立体控制级联反应,然后进行碳cup合和共轭加成反应,可解决初始手性的全部恢复问题。研究了反应的范围和局限性。环化步骤中的高非对立面辨别力允许构建具有出色的dr和ee的四元立体中心,其相反的配置取决于E-或Z-起始原料中烯烃的构型。茚的后功能化允许合成包含四个连续的立体中心的茚满基衍生物。
更新日期:2015-10-20
中文翻译:

共轭二烯-炔的有机铜引发环化:茚的非对映和对映选择性合成
有机铜试剂与容易获得的手性共轭二烯-炔反应,得到带有两个立体异构中心的茚衍生物。在光学纯系列中对该原始反应的研究表明,手性的双重转移正在起作用。涉及S N 2'的立体控制级联反应,然后进行碳cup合和共轭加成反应,可解决初始手性的全部恢复问题。研究了反应的范围和局限性。环化步骤中的高非对立面辨别力允许构建具有出色的dr和ee的四元立体中心,其相反的配置取决于E-或Z-起始原料中烯烃的构型。茚的后功能化允许合成包含四个连续的立体中心的茚满基衍生物。































 京公网安备 11010802027423号
京公网安备 11010802027423号