当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling the Reactivity of the Boronyl Group in Platinum Complexes toward Cyclodimerization: A Theoretical Survey
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2015-10-16 00:00:00 , DOI: 10.1021/acs.inorgchem.5b01597 Zhong Zhang 1, 2 , Liang Pu 1 , Qian-shu Li 2 , R. Bruce King 3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2015-10-16 00:00:00 , DOI: 10.1021/acs.inorgchem.5b01597 Zhong Zhang 1, 2 , Liang Pu 1 , Qian-shu Li 2 , R. Bruce King 3
Affiliation
|
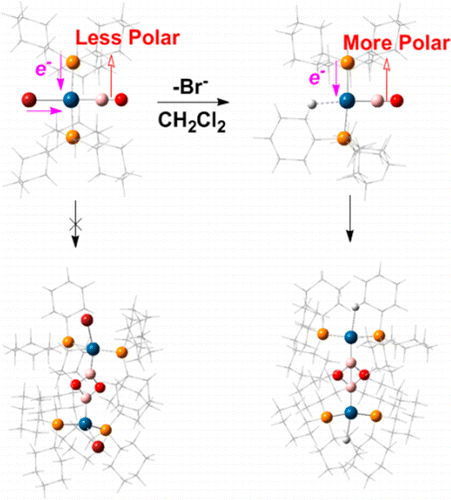
A theoretical study of the cyclodimerization of (Cy3P)2Pt(BO)Br (1Br) and [(Cy3P)2Pt(BO)]+ (1) (Cy = cyclohexyl) suggests that the reactivity of the BO ligand is primarily controlled by M←BO σ donation. Therefore, increasing the electron density at the metal center through strong σ-donor and weak π-acceptor ancillary ligands and a low formal metal oxidation state are suggested to reduce the polarity of the boronyl ligand and thus lower its reactivity toward cyclodimerization. The stable 1Br has lower Pt←BO σ donation and thus a less electrophilic boron atom, leading to a less polarized BO ligand. However, 1 is unstable in dichloromethane, since the dicationic dimer and transition state are highly stabilized by strong electrostatic interactions.
中文翻译:

控制铂络合物中的硼基对环二聚反应的反应性:理论研究。
对(Cy 3 P)2 Pt(BO)Br(1Br)和[(Cy 3 P)2 Pt(BO)] +(1)(Cy =环己基)进行环二聚的理论研究表明,BO的反应性配体主要由M←BOσ捐赠控制。因此,建议通过强的σ-给体和弱的π-受体辅助配体以及低的形式金属氧化态来增加金属中心的电子密度,以降低硼酰基配体的极性,从而降低其对环二聚反应的反应性。稳定的1Br具有较低的Pt←BOσ供体,因此具有较少的亲电硼原子,从而导致极化的BO配体较少。但是1 由于在强力的静电作用下,双官能的二聚体和过渡态高度稳定,因此在二氯甲烷中是不稳定的。
更新日期:2015-10-16
中文翻译:

控制铂络合物中的硼基对环二聚反应的反应性:理论研究。
对(Cy 3 P)2 Pt(BO)Br(1Br)和[(Cy 3 P)2 Pt(BO)] +(1)(Cy =环己基)进行环二聚的理论研究表明,BO的反应性配体主要由M←BOσ捐赠控制。因此,建议通过强的σ-给体和弱的π-受体辅助配体以及低的形式金属氧化态来增加金属中心的电子密度,以降低硼酰基配体的极性,从而降低其对环二聚反应的反应性。稳定的1Br具有较低的Pt←BOσ供体,因此具有较少的亲电硼原子,从而导致极化的BO配体较少。但是1 由于在强力的静电作用下,双官能的二聚体和过渡态高度稳定,因此在二氯甲烷中是不稳定的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号