Tetrahedron ( IF 2.1 ) Pub Date : 2018-01-09 , DOI: 10.1016/j.tet.2018.01.011 Alex A. Hunt-Painter , Bridget L. Stocker , Mattie S.M. Timmer
|
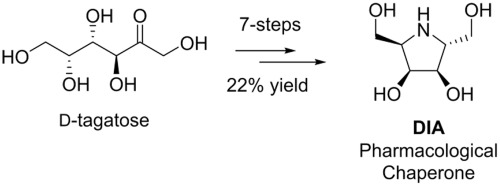
The iminosugar 2,5-dideoxy-2,5-imino-d-altritol (DIA, 2) is a powerful competitive inhibitor of α-galactosidase A and has shown much promise as a pharmacological chaperone for the treatment for Fabry disease. Notwithstanding, syntheses of DIA are in want of optimisation. Accordingly, we report on the total synthesis of DIA in 7 steps and in 22% overall yield from readily available d-tagatose. This is the shortest and most efficient synthesis of DIA to date, with key steps in our synthetic strategy including a diastereoselective reductive amination and an I2-mediated carbamate annulation.
中文翻译:

通过非对映选择性还原胺化和氨基甲酸酯环化反应合成分子伴侣2,5-二脱氧-2,5-亚氨基-d-麦芽糖醇
所述亚氨基糖-2,5-二脱氧-2,5-亚氨基d -altritol(DIA,2)是α半乳糖苷酶A的强有力的竞争抑制剂,并表现出很大希望作为治疗法布里病药理学伴侣。尽管如此,DIA的合成仍需要优化。因此,我们报告了7个步骤中DIA的总合成以及从容易获得的d-塔格糖中获得22%的总收率。这是迄今为止DIA最短,最有效的合成方法,是我们合成策略中的关键步骤,包括非对映选择性还原胺化和I 2介导的氨基甲酸酯环化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号