当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inactivation of 4-Oxalocrotonate Tautomerase by 5-Halo-2-hydroxy-2,4-pentadienoates.
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-24 , DOI: 10.1021/acs.biochem.7b00899 Tyler M M Stack 1, 2 , Wenzong Li 1, 2 , William H Johnson 3 , Yan Jessie Zhang 1, 2 , Christian P Whitman 3
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-24 , DOI: 10.1021/acs.biochem.7b00899 Tyler M M Stack 1, 2 , Wenzong Li 1, 2 , William H Johnson 3 , Yan Jessie Zhang 1, 2 , Christian P Whitman 3
Affiliation
|
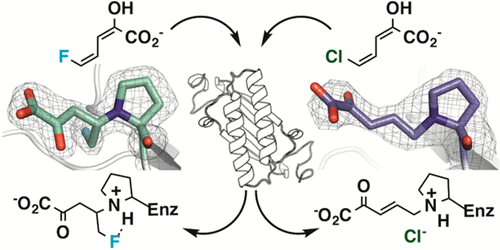
5-Halo-2-hydroxy-2,4-pentadienoates (5-halo-HPDs) are reportedly generated in the bacterial catabolism of halogenated aromatic hydrocarbons by the meta-fission pathway. The 5-halo-HPDs, where the halogen can be bromide, chloride, or fluoride, result in the irreversible inactivation of 4-oxalocrotonate tautomerase (4-OT), which precedes the enzyme that generates them. The loss of activity is due to the covalent modification of the nucleophilic amino-terminal proline. Mass spectral and crystallographic analysis of the modified enzymes indicates that inactivation of 4-OT by 5-chloro- and 5-bromo-2-hydroxy-2,4-pentadienoate follows a mechanism different from that for the inactivation of 4-OT by 5-fluoro-2-hydroxy-2,4-pentadienoate. The 5-chloro and 5-bromo derivatives undergo 4-OT-catalyzed tautomerization to their respective α,β-unsaturated ketones followed by attack at C5 (by the prolyl nitrogen) with concomitant loss of the halide. For the 5-fluoro species, the presence of a small amount of the α,β-unsaturated ketone could result in a Michael addition of the prolyl nitrogen to C4 followed by protonation at C3. The fluoride is not eliminated. These observations suggest that the inactivation of 4-OT by a downstream metabolite could hamper the efficacy of the pathway, which is the first time that such a bottleneck has been reported for the meta-fission pathway.
中文翻译:

5-卤代-2-羟基-2,4-戊二烯酸酯使4-羟基巴豆酸互变异构酶失活。
据报道,通过间裂变途径在卤代芳烃的细菌分解代谢中产生了5-卤-2-羟基-2,4-戊二烯酸酯(5-卤代-HPDs)。5-卤代HPD(其中的卤素可以是溴离子,氯离子或氟离子)会导致4-草酸巴豆酸酯互变异构酶(4-OT)的不可逆失活,该酶先于生成它们的酶。活性丧失是由于亲核氨基末端脯氨酸的共价修饰。修饰酶的质谱和晶体学分析表明,5-氯-和5-溴-2-羟基-2,4-戊二烯酸酯对4-OT的失活机制与5-HT和5-溴-2-羟基-2,4-戊二烯酸酯的失活不同。 -氟-2-羟基-2,4-戊二烯酸酯。5-氯和5-溴代衍生物会进行4-OT催化的互变异构化为各自的α,β-不饱和酮,随后在C5处(被脯氨酰氮)侵蚀,并伴随卤化物的损失。对于5-氟物质,少量α,β-不饱和酮的存在可能导致脯氨酰氮迈克尔加成到C4,然后在C3质子化。氟化物没有消除。这些观察结果表明,下游代谢物使4-OT失活可能会阻碍该途径的功效,这是首次报道了该裂变途径的瓶颈。
更新日期:2018-01-24
中文翻译:

5-卤代-2-羟基-2,4-戊二烯酸酯使4-羟基巴豆酸互变异构酶失活。
据报道,通过间裂变途径在卤代芳烃的细菌分解代谢中产生了5-卤-2-羟基-2,4-戊二烯酸酯(5-卤代-HPDs)。5-卤代HPD(其中的卤素可以是溴离子,氯离子或氟离子)会导致4-草酸巴豆酸酯互变异构酶(4-OT)的不可逆失活,该酶先于生成它们的酶。活性丧失是由于亲核氨基末端脯氨酸的共价修饰。修饰酶的质谱和晶体学分析表明,5-氯-和5-溴-2-羟基-2,4-戊二烯酸酯对4-OT的失活机制与5-HT和5-溴-2-羟基-2,4-戊二烯酸酯的失活不同。 -氟-2-羟基-2,4-戊二烯酸酯。5-氯和5-溴代衍生物会进行4-OT催化的互变异构化为各自的α,β-不饱和酮,随后在C5处(被脯氨酰氮)侵蚀,并伴随卤化物的损失。对于5-氟物质,少量α,β-不饱和酮的存在可能导致脯氨酰氮迈克尔加成到C4,然后在C3质子化。氟化物没有消除。这些观察结果表明,下游代谢物使4-OT失活可能会阻碍该途径的功效,这是首次报道了该裂变途径的瓶颈。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号