当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual Allosteric Inhibition of SHP2 Phosphatase
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acschembio.7b00980 Michelle Fodor 1 , Edmund Price 1 , Ping Wang 1 , Hengyu Lu 1 , Andreea Argintaru 1 , Zhouliang Chen 1 , Meir Glick 1 , Huai-Xiang Hao 1 , Mitsunori Kato 1 , Robert Koenig 1 , Jonathan R. LaRochelle 2 , Gang Liu 1 , Eric McNeill 1 , Dyuti Majumdar 1 , Gisele A. Nishiguchi 1 , Lawrence B. Perez 1 , Gregory Paris 1 , Christopher M. Quinn 1 , Timothy Ramsey 1 , Martin Sendzik 1 , Michael David Shultz 1 , Sarah L. Williams 1 , Travis Stams 1 , Stephen C. Blacklow 2 , Michael G. Acker 1 , Matthew J. LaMarche 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acschembio.7b00980 Michelle Fodor 1 , Edmund Price 1 , Ping Wang 1 , Hengyu Lu 1 , Andreea Argintaru 1 , Zhouliang Chen 1 , Meir Glick 1 , Huai-Xiang Hao 1 , Mitsunori Kato 1 , Robert Koenig 1 , Jonathan R. LaRochelle 2 , Gang Liu 1 , Eric McNeill 1 , Dyuti Majumdar 1 , Gisele A. Nishiguchi 1 , Lawrence B. Perez 1 , Gregory Paris 1 , Christopher M. Quinn 1 , Timothy Ramsey 1 , Martin Sendzik 1 , Michael David Shultz 1 , Sarah L. Williams 1 , Travis Stams 1 , Stephen C. Blacklow 2 , Michael G. Acker 1 , Matthew J. LaMarche 1
Affiliation
|
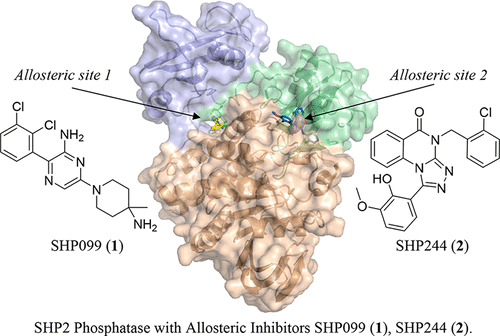
SHP2 is a cytoplasmic protein tyrosine phosphatase encoded by the PTPN11 gene and is involved in cell proliferation, differentiation, and survival. Recently, we reported an allosteric mechanism of inhibition that stabilizes the auto-inhibited conformation of SHP2. SHP099 (1) was identified and characterized as a moderately potent, orally bioavailable, allosteric small molecule inhibitor, which binds to a tunnel-like pocket formed by the confluence of three domains of SHP2. In this report, we describe further screening strategies that enabled the identification of a second, distinct small molecule allosteric site. SHP244 (2) was identified as a weak inhibitor of SHP2 with modest thermal stabilization of the enzyme. X-ray crystallography revealed that 2 binds and stabilizes the inactive, closed conformation of SHP2, at a distinct, previously unexplored binding site—a cleft formed at the interface of the N-terminal SH2 and PTP domains. Derivatization of 2 using structure-based design resulted in an increase in SHP2 thermal stabilization, biochemical inhibition, and subsequent MAPK pathway modulation. Downregulation of DUSP6 mRNA, a downstream MAPK pathway marker, was observed in KYSE-520 cancer cells. Remarkably, simultaneous occupation of both allosteric sites by 1 and 2 was possible, as characterized by cooperative biochemical inhibition experiments and X-ray crystallography. Combining an allosteric site 1 inhibitor with an allosteric site 2 inhibitor led to enhanced pharmacological pathway inhibition in cells. This work illustrates a rare example of dual allosteric targeted protein inhibition, demonstrates screening methodology and tactics to identify allosteric inhibitors, and enables further interrogation of SHP2 in cancer and related pathologies.
中文翻译:

SHP2磷酸酶的双重变构抑制作用
SHP2是由PTPN11基因编码的胞质蛋白酪氨酸磷酸酶,参与细胞增殖,分化和存活。最近,我们报道了一种抑制变构机制,该机制稳定了SHP2的自抑制构象。SHP099(1)被鉴定并表征为中等有效的,口服生物利用的,变构小分子抑制剂,该抑制剂与SHP2的三个结构域汇合形成的隧道状袋结合。在本报告中,我们描述了进一步的筛选策略,这些策略能够识别第二个不同的小分子变构位点。SHP244(2)被确定为SHP2的弱抑制剂,具有适度的酶热稳定性。X射线晶体学表明2在一个独特的,先前未开发的结合位点(在N末端SH2和PTP结构域的界面处形成的裂口)结合并稳定了SHP2的无活性,闭合构象。使用基于结构的设计2的衍生化导致SHP2热稳定性,生化抑制和随后的MAPK途径调节增加。在KYSE-520癌细胞中观察到下游MAPK通路标志物DUSP6 mRNA的下调。值得注意的是,两个变构位点同时占据了1和2以协同生化抑制实验和X射线晶体学为特征,这是可能的。将变构位点1抑制剂与变构位点2抑制剂组合导致细胞中增强的药理途径抑制作用。这项工作说明了双重变构靶向蛋白质抑制的罕见例子,展示了鉴定变构抑制剂的筛选方法和策略,并可以进一步研究SHP2在癌症和相关病理学中的作用。
更新日期:2018-01-05
中文翻译:

SHP2磷酸酶的双重变构抑制作用
SHP2是由PTPN11基因编码的胞质蛋白酪氨酸磷酸酶,参与细胞增殖,分化和存活。最近,我们报道了一种抑制变构机制,该机制稳定了SHP2的自抑制构象。SHP099(1)被鉴定并表征为中等有效的,口服生物利用的,变构小分子抑制剂,该抑制剂与SHP2的三个结构域汇合形成的隧道状袋结合。在本报告中,我们描述了进一步的筛选策略,这些策略能够识别第二个不同的小分子变构位点。SHP244(2)被确定为SHP2的弱抑制剂,具有适度的酶热稳定性。X射线晶体学表明2在一个独特的,先前未开发的结合位点(在N末端SH2和PTP结构域的界面处形成的裂口)结合并稳定了SHP2的无活性,闭合构象。使用基于结构的设计2的衍生化导致SHP2热稳定性,生化抑制和随后的MAPK途径调节增加。在KYSE-520癌细胞中观察到下游MAPK通路标志物DUSP6 mRNA的下调。值得注意的是,两个变构位点同时占据了1和2以协同生化抑制实验和X射线晶体学为特征,这是可能的。将变构位点1抑制剂与变构位点2抑制剂组合导致细胞中增强的药理途径抑制作用。这项工作说明了双重变构靶向蛋白质抑制的罕见例子,展示了鉴定变构抑制剂的筛选方法和策略,并可以进一步研究SHP2在癌症和相关病理学中的作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号