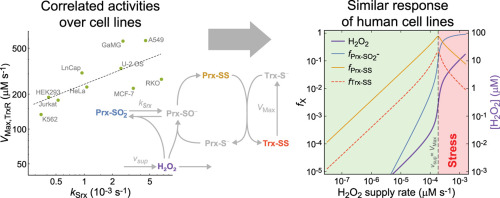
由典型的 2-Cys 过氧化还原蛋白 (Prx)、硫氧还蛋白 (Trx)、Trx 还原酶 (TrxR) 和硫氧还蛋白 (Srx) 形成的系统 (PTTRS) 是真核细胞细胞质中抗氧化保护和氧化还原信号传导的核心。了解 PTTRS 如何整合这些功能需要将表型追踪到分子特性,这并非易事。在这里,我们基于捕获 PTTRS 保守特征的模型来分析这个问题。我们绘制了对 H 2 O 2供应率 ( v support )产生每种不同反应的条件,并估计了 13 种人类细胞类型和酿酒酵母的参数。由此产生的组成到表型图谱产生了以下可通过实验测试的预测。PTTRS 允许许多不同的响应,包括超灵敏度和滞后。然而,几乎所有肿瘤细胞系都表现出类似的反应,其特征是有限的Trx-S消耗和在高vsup时大量但自限性的过度氧化的Prx逐渐积累。这种相似性源于细胞系中 TrxR、Srx 和 Prx 活性之间的强相关性,这有助于保持 Prx-SS 还原能力略高于最大稳态 Prx-SS 产量。反过来,在红细胞、肝细胞和 HepG2 细胞中,高电压消耗Trx-S -并将 Prx 主要氧化为 Prx-SS。在所有有核人类细胞中,Prx-SS 还原能力定义了分隔两种不同状态的阈值。在阈值下vsup处,细胞质H 2 O 2浓度由Prx、nM范围和空间局部化决定,而在阈值上vsup处,细胞质H 2 O 2浓度由整个细胞质中活性低得多的替代库和μM范围决定。当 Prx Tsa1 在高v support下以磺酸盐形式积累时,酵母表现出明显的反应。这主要是由于 Tsa1 的磺酸盐具有出色的稳定性。讨论了这些发现对硫醇氧化还原调节和细胞生理学的影响。所有估计值都经过彻底记录和提供,以及系统特性的分析近似值,作为定量氧化还原生物学的资源。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Mapping the phenotypic repertoire of the cytoplasmic 2-Cys peroxiredoxin – Thioredoxin system. 1. Understanding commonalities and differences among cell types
The system (PTTRS) formed by typical 2-Cys peroxiredoxins (Prx), thioredoxin (Trx), Trx reductase (TrxR), and sulfiredoxin (Srx) is central in antioxidant protection and redox signaling in the cytoplasm of eukaryotic cells. Understanding how the PTTRS integrates these functions requires tracing phenotypes to molecular properties, which is non-trivial. Here we analyze this problem based on a model that captures the PTTRS’ conserved features. We have mapped the conditions that generate each distinct response to H2O2 supply rates (vsup), and estimated the parameters for thirteen human cell types and for Saccharomyces cerevisiae. The resulting composition-to-phenotype map yielded the following experimentally testable predictions. The PTTRS permits many distinct responses including ultra-sensitivity and hysteresis. However, nearly all tumor cell lines showed a similar response characterized by limited Trx-S- depletion and a substantial but self-limited gradual accumulation of hyperoxidized Prx at high vsup. This similarity ensues from strong correlations between the TrxR, Srx and Prx activities over cell lines, which contribute to maintain the Prx-SS reduction capacity in slight excess over the maximal steady state Prx-SS production. In turn, in erythrocytes, hepatocytes and HepG2 cells high vsup depletes Trx-S- and oxidizes Prx mainly to Prx-SS. In all nucleated human cells the Prx-SS reduction capacity defined a threshold separating two different regimes. At sub-threshold vsup the cytoplasmic H2O2 concentration is determined by Prx, nM-range and spatially localized, whereas at supra-threshold vsup it is determined by much less active alternative sinks and μM-range throughout the cytoplasm. The yeast shows a distinct response where the Prx Tsa1 accumulates in sulfenate form at high vsup. This is mainly due to an exceptional stability of Tsa1's sulfenate. The implications of these findings for thiol redox regulation and cell physiology are discussed. All estimates were thoroughly documented and provided, together with analytical approximations for system properties, as a resource for quantitative redox biology.


































 京公网安备 11010802027423号
京公网安备 11010802027423号