当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Z‐Scheme Photocatalytic Water Splitting on a 2D Heterostructure of Black Phosphorus/Bismuth Vanadate Using Visible Light
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201711357 Mingshan Zhu 1 , Zhichao Sun 2 , Mamoru Fujitsuka 1 , Tetsuro Majima 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201711357 Mingshan Zhu 1 , Zhichao Sun 2 , Mamoru Fujitsuka 1 , Tetsuro Majima 1
Affiliation
|
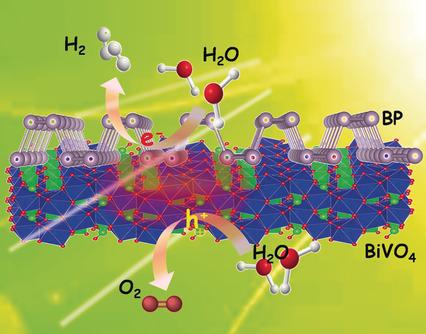
Spontaneously solar‐driven water splitting to produce H2 and O2, that is, the conversion of solar energy to chemical energy is a dream of mankind. However, it is difficult to make overall water splitting feasible without using any sacrificial agents and external bias. Drawing inspiration from nature, a new artificial Z‐scheme photocatalytic system has been designed herein based on the two‐dimensional (2D) heterostructure of black phosphorus (BP)/bismuth vanadate (BiVO4). An effective charge separation makes possible the reduction and oxidation of water on BP and BiVO4, respectively. The optimum H2 and O2 production rates on BP/BiVO4 were approximately 160 and 102 μmol g−1 h−1 under irradiation of light with a wavelength longer than 420 nm, without using any sacrificial agents or external bias.
中文翻译:

可见光在黑色磷/钒酸铋的二维异质结构上进行Z方案光催化水分解
自发地由太阳能驱动的水分解产生H 2和O 2,即将太阳能转化为化学能是人类的梦想。但是,在不使用任何牺牲剂和外部偏压的情况下,很难使整个水分解可行。从自然界中汲取灵感,本文基于黑磷(BP)/钒酸铋(BiVO 4)的二维(2D)异质结构设计了一种新型的人工Z方案光催化系统。有效的电荷分离使得分别在BP和BiVO 4上还原和氧化水成为可能。BP / BiVO 4的最佳H 2和O 2生产率在不使用任何牺牲剂或外部偏压的情况下,在波长大于420 nm的光照射下,它们分别约为160和102μmolg -1 h -1。
更新日期:2018-01-09
中文翻译:

可见光在黑色磷/钒酸铋的二维异质结构上进行Z方案光催化水分解
自发地由太阳能驱动的水分解产生H 2和O 2,即将太阳能转化为化学能是人类的梦想。但是,在不使用任何牺牲剂和外部偏压的情况下,很难使整个水分解可行。从自然界中汲取灵感,本文基于黑磷(BP)/钒酸铋(BiVO 4)的二维(2D)异质结构设计了一种新型的人工Z方案光催化系统。有效的电荷分离使得分别在BP和BiVO 4上还原和氧化水成为可能。BP / BiVO 4的最佳H 2和O 2生产率在不使用任何牺牲剂或外部偏压的情况下,在波长大于420 nm的光照射下,它们分别约为160和102μmolg -1 h -1。































 京公网安备 11010802027423号
京公网安备 11010802027423号