当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Et3N mediated synthesis of polysubstituted thiophenes from α-oxo ketene dithioacetals
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2015-10-14 15:53:27
Xiaoli Kan, Xiaobing Yang, Fangzhong Hu, Yang Wang, Ying Liu, Xiaomao Zou, Hengyu Li, Hao Li, Qichun Zhang
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2015-10-14 15:53:27
Xiaoli Kan, Xiaobing Yang, Fangzhong Hu, Yang Wang, Ying Liu, Xiaomao Zou, Hengyu Li, Hao Li, Qichun Zhang
|
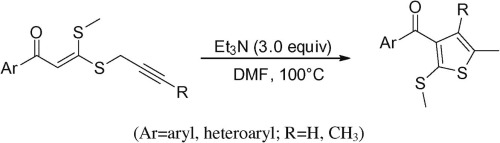
In this Letter, an efficient synthetic route to prepare polysubstituted thiophene derivatives was achieved via the Et3N-mediated (Claisen) rearrangement reaction from α-oxo S-methyl-S-propargyl ketenes, which were obtained through alkylation of β-oxodithioesters with propargylic bromides, followed by regioselective intramolecular cyclization.
中文翻译:

Et3N介导的α-氧代乙烯酮二硫缩醛合成多取代噻吩
在这封信中,通过Et 3 N介导的α-氧代S-甲基-S-炔丙基烯酮的Et 3 N介导的(克莱森)重排反应,获得了制备多取代噻吩衍生物的有效合成路线,该反应是通过将β-氧代二硫代酯与炔丙基溴,然后进行区域选择性分子内环化。
更新日期:2015-10-15
中文翻译:

Et3N介导的α-氧代乙烯酮二硫缩醛合成多取代噻吩
在这封信中,通过Et 3 N介导的α-氧代S-甲基-S-炔丙基烯酮的Et 3 N介导的(克莱森)重排反应,获得了制备多取代噻吩衍生物的有效合成路线,该反应是通过将β-氧代二硫代酯与炔丙基溴,然后进行区域选择性分子内环化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号