Developmental Cell ( IF 10.7 ) Pub Date : 2017-12-21 , DOI: 10.1016/j.devcel.2017.11.020 Kirill Bersuker , Clark W.H. Peterson , Milton To , Steffen J. Sahl , Victoria Savikhin , Elizabeth A. Grossman , Daniel K. Nomura , James A. Olzmann
|
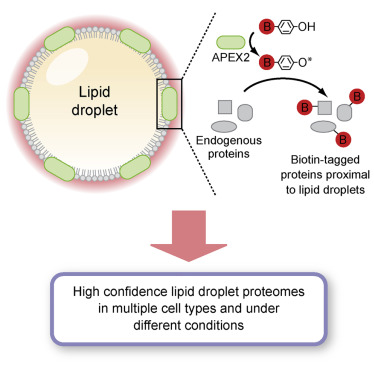
Lipid droplet (LD) functions are regulated by a complement of integral and peripheral proteins that associate with the bounding LD phospholipid monolayer. Defining the composition of the LD proteome has remained a challenge due to the presence of contaminating proteins in LD-enriched buoyant fractions. To overcome this limitation, we developed a proximity labeling strategy that exploits LD-targeted APEX2 to biotinylate LD proteins in living cells. Application of this approach to two different cell types identified the vast majority of previously validated LD proteins, excluded common contaminating proteins, and revealed new LD proteins. Moreover, quantitative analysis of LD proteome dynamics uncovered a role for endoplasmic reticulum-associated degradation in controlling the composition of the LD proteome. These data provide an important resource for future LD studies and demonstrate the utility of proximity labeling to study the regulation of LD proteomes.
中文翻译:

邻近标记策略可提供有关脂质液滴蛋白质组学组成和动力学的见解
脂质小滴(LD)的功能由与结合的LD磷脂单层缔合的整合蛋白和外围蛋白的补体调节。由于在富含LD的浮力级分中存在污染蛋白,因此定义LD蛋白质组的组成仍然是一个挑战。为了克服此限制,我们开发了一种接近标记策略,该策略利用以LD为目标的APEX2对生物蛋白在活细胞中进行生物素化。将该方法应用于两种不同的细胞类型,可鉴定出大多数先前已验证的LD蛋白,排除了常见的污染蛋白,并揭示了新的LD蛋白。此外,LD蛋白质组动力学的定量分析揭示了内质网相关降解在控制LD蛋白质组组成中的作用。





























 京公网安备 11010802027423号
京公网安备 11010802027423号