当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidation of Isomerization Pathways of a Single Azobenzene Derivative Using an STM
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2015-10-12 00:00:00 , DOI: 10.1021/acs.jpclett.5b01847 Emiko Kazuma 1 , Mina Han 2 , Jaehoon Jung 1 , Junepyo Oh 1 , Takahiro Seki 2 , Yousoo Kim 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2015-10-12 00:00:00 , DOI: 10.1021/acs.jpclett.5b01847 Emiko Kazuma 1 , Mina Han 2 , Jaehoon Jung 1 , Junepyo Oh 1 , Takahiro Seki 2 , Yousoo Kim 1
Affiliation
|
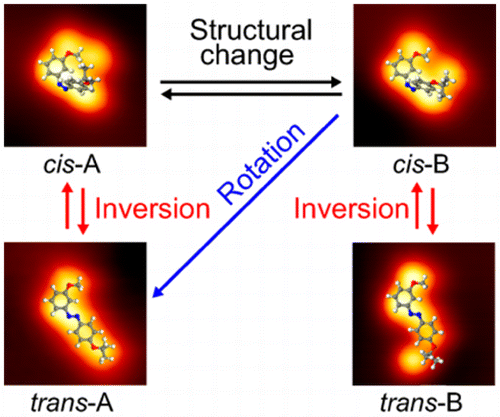
The predominant pathway for the isomerization between cis- and trans-azobenzenes—either (i) inversion by the bending of an NNC bond or (ii) rotation by the torsion of two phenyl rings—continues to be a controversial topic. To elucidate each isomerization pathway, a strategically designed and synthesized azobenzene derivative was investigated on a Ag(111) surface. This was achieved by exciting the molecule with tunneling electrons from the tip of a scanning tunneling microscope (STM). Structural analyses of the molecularly resolved STM images reveal that both inversion and rotation pathways are available for isomerization on a metal surface and strongly depend on the initial adsorption structures of the molecule. On the basis of the potential energy diagrams for the isomerization, it is concluded that isomerization pathways on a metal surface are not simply related to the excited states.
中文翻译:

使用STM阐明单一偶氮苯衍生物的异构化途径
顺式和反式之间异构化的主要途径。-偶氮苯(i)通过NNC键的弯曲而反转或(ii)通过两个苯环的扭转而旋转-仍然是一个有争议的话题。为了阐明每个异构化途径,在Ag(111)表面上研究了策略设计和合成的偶氮苯衍生物。这是通过用来自扫描隧道显微镜(STM)尖端的隧道电子激发分子来实现的。分子分辨STM图像的结构分析表明,转化和旋转途径均可用于金属表面上的异构化,并且在很大程度上取决于分子的初始吸附结构。根据异构化的势能图,可以得出结论,金属表面上的异构化途径不仅与激发态有关。
更新日期:2015-10-12
中文翻译:

使用STM阐明单一偶氮苯衍生物的异构化途径
顺式和反式之间异构化的主要途径。-偶氮苯(i)通过NNC键的弯曲而反转或(ii)通过两个苯环的扭转而旋转-仍然是一个有争议的话题。为了阐明每个异构化途径,在Ag(111)表面上研究了策略设计和合成的偶氮苯衍生物。这是通过用来自扫描隧道显微镜(STM)尖端的隧道电子激发分子来实现的。分子分辨STM图像的结构分析表明,转化和旋转途径均可用于金属表面上的异构化,并且在很大程度上取决于分子的初始吸附结构。根据异构化的势能图,可以得出结论,金属表面上的异构化途径不仅与激发态有关。





























 京公网安备 11010802027423号
京公网安备 11010802027423号