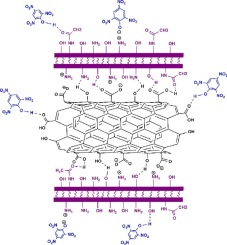
已经研究了用壳聚糖(Chi)修饰羧化多壁碳纳米管(MWCNT-COOH)以制备用于从水溶液中去除苦味酸的纳米复合材料(MWCNT-Chi)。通过FT-IR,TGA,DTG,FESEM,EDX,BET和ζ电位对材料进行了表征。通过分批实验研究溶液的pH值,吸附剂的用量,接触时间,苦味酸的浓度和温度,以研究吸附过程。动力学研究通过伪二级动力学模型对两种吸附剂进行了很好的描述。六个等温模型:Langmuir(四种线性形式),Freundlich,Tempkin,Halsey,Harkins-Jura和Dubinin-Radushkevich模型用于确定吸附过程的特征参数。等温线研究表明,两种吸附剂的MWCNT-Chi和Freundlich和Halsey模型的Langmuir等温线最能代表测得的吸附数据。另外,Dubinin-Radushkevich模型的结果证实了物理吸附。MWCNT-Chi的负ΔG°值和MWCNT-COOH的正ΔG°值分别表明在所研究浓度范围内吸附过程的自发性和不自发性。此外,在pH = 9时,苦味酸分子可从MWCNT-Chi解吸高达90%,并且消耗的MWCNT-Chi可在再生的第5个循环之前被重新利用。MWCNT-Chi的负ΔG°值和MWCNT-COOH的正ΔG°值分别表明在所研究浓度范围内吸附过程的自发性和不自发性。此外,在pH = 9时,苦味酸分子可从MWCNT-Chi解吸高达90%,并且消耗的MWCNT-Chi可在再生的第5个循环之前被重新利用。MWCNT-Chi的负ΔG°值和MWCNT-COOH的正ΔG°值分别表明在所研究浓度范围内吸附过程的自发性和不自发性。此外,在pH = 9时,苦味酸分子可从MWCNT-Chi解吸高达90%,并且消耗的MWCNT-Chi可在再生的第5个循环之前被重新利用。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Synthesis, characterization and study of sorption parameters of multi-walled carbon nanotubes/chitosan nanocomposite for the removal of picric acid from aqueous solutions
The modification of carboxylated multi-wall carbon nanotubes (MWCNT-COOH) with chitosan (Chi) has been investigated to prepare a nanocomposite material (MWCNT-Chi) for the removal of picric acid from aqueous solutions. Materials were characterized by FT-IR, TGA, DTG, FESEM, EDX, BET and zeta potential. Batch experiments such as solution pH, dosage of adsorbents, contact time, concentration of the picric acid and temperature were achieved to study sorption process. Kinetic studies were well described by pseudo-second-order kinetic model for both adsorbents. The six isotherm models: Langmuir (four linear forms), Freundlich, Tempkin, Halsey, Harkins-Jura and Dubinin-Radushkevich models were applied to determine the characteristic parameters of the adsorption process. Isotherm studies showed that the Langmuir isotherm for MWCNT-Chi and Freundlich and Halsey models for both adsorbents were found to best represent the measured sorption data. In addition, the results of Dubinin-Radushkevich model confirmed the physical adsorption. Negative ΔG° values for MWCNT-Chi and positive ones for MWCNT-COOH indicated the nature of spontaneous and unspontaneous, respectively for adsorption process in the range of the studied concentrations. In addition, picric acid molecules can be desorbed from MWCNT-Chi up to 90% at pH = 9 and that the consumed MWCNT-Chi could be reutilized up to 5th cycle of regeneration.


































 京公网安备 11010802027423号
京公网安备 11010802027423号