当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High Resolution Crystal Structures of the Acetohydroxyacid Synthase‐Pyruvate Complex Provide New Insights into Its Catalytic Mechanism
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-12-21 , DOI: 10.1002/slct.201702128
Thierry Lonhienne 1 , Mario D. Garcia 1 , Chris Noble 2 , Jeffrey Harmer 2 , James A. Fraser 1 , Craig M. Williams 1 , Luke W. Guddat 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-12-21 , DOI: 10.1002/slct.201702128
Thierry Lonhienne 1 , Mario D. Garcia 1 , Chris Noble 2 , Jeffrey Harmer 2 , James A. Fraser 1 , Craig M. Williams 1 , Luke W. Guddat 1
Affiliation
|
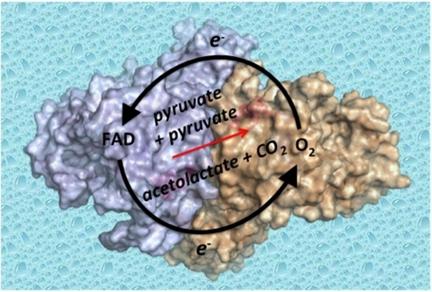
Acetohydroxyacid synthase (AHAS) is the first enzyme in the biosynthesis pathway of the branched‐chain amino acids, catalyzing the condensation of pyruvate with another molecule of pyruvate or with 2‐ketobutyrate, to produce 2‐acetolactate or 2‐acetohydroxybutyrate, respectively. The catalytic subunit of the dimeric enzyme has thiamin diphosphate (ThDP), a divalent metal ion, flavin adenine dinucleotide (FAD), and two molecules of oxygen (O2(I) and O2(II)) as cofactors. Here, crystal structures of Saccharomyces cerevisiae AHAS in complex with pyruvate provide novel insights into the mechanistic features of this enzyme including: i) The precise position taken by pyruvate molecules as they enter the active site (i. e. prior to catalysis occurring) with conformations suitable for the transfer of electrons to/from O2(I) and FAD; ii) The formation of ternary donor‐acceptor‐O2(I) complexes and iii) The location of O2(II) relative to the substrate showing that it plays a critical role in the organization of substrate for catalysis. These structural data, accompanied by electron paramagnetic resonance evidence that a radical is produced during AHAS catalysis, lead to the proposal that FAD and O2 are involved in an indirect one‐electron redox cycle. In this mechanism, the spatial configurations of O2 and FAD in the active site can allow electrons to be exchanged with the substrates and catalytic intermediates to satisfy and control the overall AHAS catalyzed reaction.
中文翻译:

乙酰羟酸合酶-丙酮酸酯复合物的高分辨率晶体结构为其催化机理提供了新的见解
乙酰羟酸合酶(AHAS)是支链氨基酸生物合成途径中的第一种酶,催化丙酮酸与另一分子丙酮酸或2-酮丁酸的缩合反应,分别生成2-乙酰丙酸或2-乙酰羟丁酸。二聚酶的催化亚基具有硫胺二磷酸(ThDP),二价金属离子,黄素腺嘌呤二核苷酸(FAD)和两个氧分子(O 2(I)和O 2(II))作为辅助因子。在这里,酿酒酵母AHAS与丙酮酸的复合物的晶体结构提供了对该酶机制特征的新见解,包括:i)丙酮酸分子进入活性位点(即发生催化之前)时所具有的精确位置,其构象适合电子往返于O 2(I)和FAD的转移;ii)三元供体-受体-O 2(I)配合物的形成,以及iii)O 2(II)相对于底物的位置表明它在催化底物的组织中起着关键作用。这些结构数据,连同电子顺磁共振证据表明,在AHAS催化过程中会产生自由基,因此提出了FAD和O 2的建议。参与间接的单电子氧化还原循环。在这种机制下,活性位点中O 2和FAD的空间构型可以使电子与底物和催化中间体进行交换,从而满足并控制整个AHAS催化的反应。
更新日期:2017-12-21
中文翻译:

乙酰羟酸合酶-丙酮酸酯复合物的高分辨率晶体结构为其催化机理提供了新的见解
乙酰羟酸合酶(AHAS)是支链氨基酸生物合成途径中的第一种酶,催化丙酮酸与另一分子丙酮酸或2-酮丁酸的缩合反应,分别生成2-乙酰丙酸或2-乙酰羟丁酸。二聚酶的催化亚基具有硫胺二磷酸(ThDP),二价金属离子,黄素腺嘌呤二核苷酸(FAD)和两个氧分子(O 2(I)和O 2(II))作为辅助因子。在这里,酿酒酵母AHAS与丙酮酸的复合物的晶体结构提供了对该酶机制特征的新见解,包括:i)丙酮酸分子进入活性位点(即发生催化之前)时所具有的精确位置,其构象适合电子往返于O 2(I)和FAD的转移;ii)三元供体-受体-O 2(I)配合物的形成,以及iii)O 2(II)相对于底物的位置表明它在催化底物的组织中起着关键作用。这些结构数据,连同电子顺磁共振证据表明,在AHAS催化过程中会产生自由基,因此提出了FAD和O 2的建议。参与间接的单电子氧化还原循环。在这种机制下,活性位点中O 2和FAD的空间构型可以使电子与底物和催化中间体进行交换,从而满足并控制整个AHAS催化的反应。







































 京公网安备 11010802027423号
京公网安备 11010802027423号