Chemosphere ( IF 8.1 ) Pub Date : 2017-12-19 , DOI: 10.1016/j.chemosphere.2017.12.113 Tianyang Zhang , Bin Xu , Anqi Wang , Changzheng Cui
|
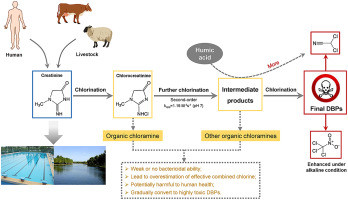
Organic chloramines can interfere with the measurement of effective combined chlorine in chlorinated water and are potential intermediate products of highly toxic disinfection by-products (DBPs). In order to know more about the degradation and transformation of organic chloramines, a typical organic chloramine precursor creatinine was selected for investigation and a corresponding individual organic chloramine chlorocreatinine was prepared in this study. The preparation condition of chlorocreatinine by chlorination was established as chlorine/creatinine = 1 M/M, reaction time = 2 h and pH = 7.0. Then the degradation kinetics of chlorocreatinine during further chlorination was studied, and a second-order rate constant of 1.16 (±0.14) M−1 s−1 was obtained at pH 7.0. Solution pH significantly influenced the degradation rate, and the elementary rate constants of chlorocreatinine with HOCl+H+, HOCl, OCl− and chlorocreatinine− with OCl− were calculated as 2.43 (±1.55) × 104 M−2 s−1, 1.05 (±0.09) M−1 s−1, 2.86 (±0.30) M−1 s−1 and 3.09 (±0.24) M−1 s−1, respectively. Besides, it was found that chlorocreatinine could be further converted into several C-DBPs (chloroform and trichloroacetone) and N-DBPs (dichloroacetonitrile (DCAN) and trichloronitromethane (TCNM)) during chlorination. The total yield of DBPs increased obviously with increasing pH, especially for TCNM. In addition, the presence of humic acid in creatinine solution could increase the formation of DCAN obviously during chlorination. Based on the UPLC-Q-TOF-MS analysis, the conversion pathways of chlorocreatinine were proposed. Several kinds of intermediate products were also identified as organic chloramines and some of them could even exist stably during the further chlorination.
中文翻译:

肌酐氯化过程中有机氯胺的降解动力学和消毒副产物的形成
有机氯胺会干扰对氯化水中有效氯含量的测量,并且是高毒性消毒副产物(DBP)的潜在中间产物。为了更多地了解有机氯胺的降解和转化,选择了典型的有机氯胺前体肌酐进行研究,并在本研究中制备了相应的有机氯胺。确定了通过氯化制备氯肌酐的条件为氯/肌酐= 1 M / M,反应时间= 2 h,pH = 7.0。然后研究了氯肌酐在进一步氯化过程中的降解动力学,其二级速率常数为1.16(±0.14)M -1 s -1在pH 7.0下获得产物。溶液pH值的影响显著的降解率,并用次氯酸+ H chlorocreatinine的基本速率常数+,次氯酸,OCL -和chlorocreatinine -与OCL -计算为2.43(±1.55)×10 4 中号-2 小号-1,1.05 (±0.09)M -1小号-1,2.86(±0.30)M -1小号-1和3.09(±0.24)M -1小号-1, 分别。此外,发现氯代肌酸酐在氯化过程中可以进一步转化为几种C-DBPs(氯仿和三氯丙酮)和N-DBPs(二氯乙腈(DCAN)和三氯硝基甲烷(TCNM))。随着pH值的升高,DBP的总收率明显增加,尤其是对于TCNM。此外,肌酐溶液中腐殖酸的存在可明显增加氯化过程中DCAN的形成。在UPLC-Q-TOF-MS分析的基础上,提出了氯肌酐的转化途径。几种中间产物也被鉴定为有机氯胺,其中一些甚至可以在进一步氯化过程中稳定存在。































 京公网安备 11010802027423号
京公网安备 11010802027423号