当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
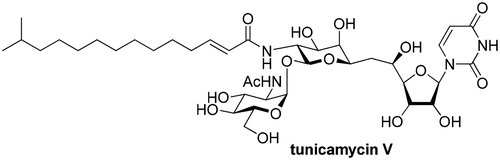
Total Synthesis of Tunicamycin V
Organic Letters ( IF 4.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.orglett.7b03623 Kazuki Yamamoto 1 , Fumika Yakushiji 1, 2 , Takanori Matsumaru 1, 2 , Satoshi Ichikawa 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.orglett.7b03623 Kazuki Yamamoto 1 , Fumika Yakushiji 1, 2 , Takanori Matsumaru 1, 2 , Satoshi Ichikawa 1, 2
Affiliation
|
The total synthesis of tunicamycin V is described. This strategy is based on the initial construction of tunicaminyluracil, which is regarded to play an important role in the observed biological activities. The key to the synthesis was a Mukaiyama aldol reaction followed by a furan-oxidation to construct the undecose skeleton, a [3,3] sigmatropic rearrangement of a cyanate, and a highly selective trehalose-type glycosylation.
中文翻译:

衣霉素V的全合成
描述了衣霉素V的全合成。该策略基于衣氨酰尿嘧啶的初始构建,该构建被认为在观察到的生物活性中起重要作用。合成的关键是Mukaiyama aldol反应,然后进行呋喃氧化以构建十一碳烯骨架,氰酸酯的[3,3]σ重排以及高度选择性的海藻糖型糖基化。
更新日期:2017-12-19
中文翻译:

衣霉素V的全合成
描述了衣霉素V的全合成。该策略基于衣氨酰尿嘧啶的初始构建,该构建被认为在观察到的生物活性中起重要作用。合成的关键是Mukaiyama aldol反应,然后进行呋喃氧化以构建十一碳烯骨架,氰酸酯的[3,3]σ重排以及高度选择性的海藻糖型糖基化。







































 京公网安备 11010802027423号
京公网安备 11010802027423号