European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-12-16 , DOI: 10.1016/j.ejmech.2017.12.057 Yan-Qiong Guo , Gui-Hua Tang , Lan-Lan Lou , Wei Li , Bei Zhang , Bo Liu , Sheng Yin
|
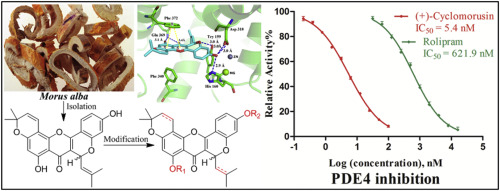
The bioassay-guided phytochemical study of a traditional Chinese medicine Morus alba led to the isolation of 18 prenylated flavonoids (1–18), of which (±)-cyclomorusin (1/2), a pair of enantiomers, and 14-methoxy-dihydromorusin (3) are the new ones. Subsequent structural modification of the selected components by methylation, esterification, hydrogenation, and oxidative cyclization led to 14 more derivatives (19–32). The small library was screened for its inhibition against phosphodiesterase-4 (PDE4), which is a drug target for the treatment of asthma and chronic obstructive pulmonary disease (COPD). Among them, nine compounds (1–5, 8, 10, 16, and 17) exhibited remarkable activities with IC50 values ranging from 0.0054 to 0.40 μM, being more active than the positive control rolipram (IC50 = 0.62 μM). (+)-Cyclomorusin (1), the most active natural PDE4 inhibitor reported so far, also showed a high selectivity across other PDE members with the selective fold greater than 55. The SAR study revealed that the presence of prenyls at C-3 and/or C-8, 2H-pyran ring D, and the phenolic hydroxyl groups were important to the activity, which was further supported by the recognition mechanism study of the inhibitors with PDE4 by using molecular modeling.






























 京公网安备 11010802027423号
京公网安备 11010802027423号