Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Functional Characterization of a Cross-Reactive Dengue Virus Neutralizing Antibody that Recognizes a Cryptic Epitope.
Structure ( IF 4.4 ) Pub Date : 2018-01-02 , DOI: 10.1016/j.str.2017.11.017 Jie Li , Daniel Watterson , Chiung-Wen Chang , Xiao-Yan Che , Xiao-Quan Li , Daniel J. Ericsson , Li-Wen Qiu , Jian-Piao Cai , Jing Chen , Scott R. Fry , Stacey T.M. Cheung , Matthew A. Cooper , Paul R. Young , Bostjan Kobe
Structure ( IF 4.4 ) Pub Date : 2018-01-02 , DOI: 10.1016/j.str.2017.11.017 Jie Li , Daniel Watterson , Chiung-Wen Chang , Xiao-Yan Che , Xiao-Quan Li , Daniel J. Ericsson , Li-Wen Qiu , Jian-Piao Cai , Jing Chen , Scott R. Fry , Stacey T.M. Cheung , Matthew A. Cooper , Paul R. Young , Bostjan Kobe
|
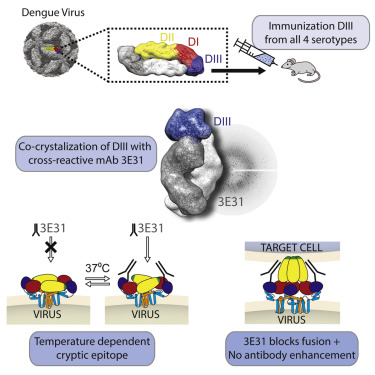
Understanding the molecular basis of the neutralizing antibody response to dengue virus (DENV) is an essential component in the design and development of effective vaccines and immunotherapeutics. Here we present the structure of a cross-reactive, neutralizing antibody, 3E31, in complex with domain III (DIII) of the DENV envelope (E) protein and reveal a conserved, temperature-sensitive, cryptic epitope on DIII that is not available in any of the known conformations of E on the dengue virion. We observed that 3E31 inhibits E-mediated membrane fusion, suggesting that the antibody is able to neutralize virus through binding an as-yet uncharacterized intermediate conformation of DENV E and sterically block trimer formation. Finally, we show that, unlike cross-reactive fusion peptide-specific antibodies, 3E31 does not promote antibody-dependent enhancement of infection at sub-neutralizing concentrations. Our results highlight the 3E31 epitope on the E protein DIII as a promising target for immunotherapeutics or vaccine design.
中文翻译:

交叉反应登革热病毒中和抗体的结构和功能表征,该抗体可识别隐秘表位。
理解对登革热病毒(DENV)的中和抗体应答的分子基础是有效疫苗和免疫疗法的设计和开发中的重要组成部分。在这里,我们介绍了与DENV包膜(E)蛋白的结构域III(DIII)结合的交叉反应,中和抗体3E31的结构,并揭示了DIII中不存在的保守的,对温度敏感的隐性表位E在登革热病毒粒子上的任何已知构象。我们观察到3E31抑制E介导的膜融合,表明该抗体能够通过结合DENV E的尚未表征的中间构象和空间阻断三聚体的形成来中和病毒。最后,我们证明,与交叉反应性融合肽特异性抗体不同,3E31在中和浓度以下时不会促进抗体依赖性感染的增强。我们的结果突出了E蛋白DIII上的3E31表位,将其作为免疫疗法或疫苗设计的有希望的靶标。
更新日期:2017-12-16
中文翻译:

交叉反应登革热病毒中和抗体的结构和功能表征,该抗体可识别隐秘表位。
理解对登革热病毒(DENV)的中和抗体应答的分子基础是有效疫苗和免疫疗法的设计和开发中的重要组成部分。在这里,我们介绍了与DENV包膜(E)蛋白的结构域III(DIII)结合的交叉反应,中和抗体3E31的结构,并揭示了DIII中不存在的保守的,对温度敏感的隐性表位E在登革热病毒粒子上的任何已知构象。我们观察到3E31抑制E介导的膜融合,表明该抗体能够通过结合DENV E的尚未表征的中间构象和空间阻断三聚体的形成来中和病毒。最后,我们证明,与交叉反应性融合肽特异性抗体不同,3E31在中和浓度以下时不会促进抗体依赖性感染的增强。我们的结果突出了E蛋白DIII上的3E31表位,将其作为免疫疗法或疫苗设计的有希望的靶标。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号