当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Cluster Catalyst for N2-to-NH3 Thermal Conversion
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-20 , DOI: 10.1021/jacs.7b10354 Xue-Lu Ma 1 , Jin-Cheng Liu 1 , Hai Xiao 1 , Jun Li 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-20 , DOI: 10.1021/jacs.7b10354 Xue-Lu Ma 1 , Jin-Cheng Liu 1 , Hai Xiao 1 , Jun Li 1
Affiliation
|
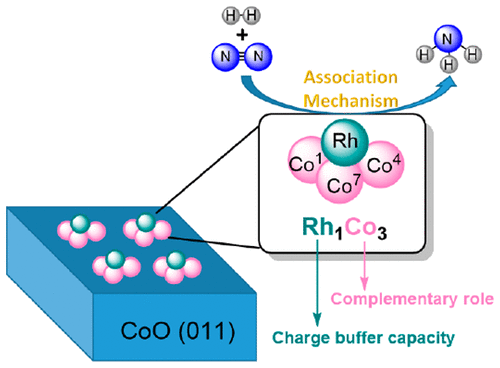
The ammonia synthesis from N2 is of vital importance, with imitating biological nitrogen fixation attracted much interest. Herein, we investigate the catalytic mechanisms of N2-to-NH3 thermal conversion on the singly dispersed bimetallic catalyst Rh1Co3/CoO(011), and find that the preferred pathway is an associative mechanism analogous to the biological process, in which alternating hydrogenations of the N2 occur, with H2 activation on both metal sites. We propose that the singly dispersed bimetallic M1An catalyst, in which the doped metal atom M substitutes an oxygen atom on the oxide surface of metal A, serves as a new surface single-cluster catalyst (SCC) design platform for the biomimetic N2-to-NH3 thermal conversion. The catalytic ability of M1An catalyst is attributed to both the charge buffer capacity of doped metal M and the complementary role of synergic metal A in catalysis. Our work provides insights and guidelines for further optimizing the M1An catalyst.
中文翻译:

用于 N2 到 NH3 热转化的表面簇催化剂
N2 的氨合成至关重要,模拟生物固氮引起了很多兴趣。在此,我们研究了单分散双金属催化剂 Rh1Co3/CoO(011) 上 N2 到 NH3 热转化的催化机制,发现首选途径是类似于生物过程的缔合机制,其中交替氢化N2 发生,H2 在两个金属位点上都被激活。我们提出单分散的双金属 M1An 催化剂,其中掺杂的金属原子 M 取代了金属 A 氧化物表面上的氧原子,作为一种新的表面单簇催化剂(SCC)设计平台,用于仿生 N2-to- NH3 热转化。M1An催化剂的催化能力归因于掺杂金属M的电荷缓冲能力和协同金属A在催化中的互补作用。我们的工作为进一步优化 M1An 催化剂提供了见解和指导。
更新日期:2017-12-20
中文翻译:

用于 N2 到 NH3 热转化的表面簇催化剂
N2 的氨合成至关重要,模拟生物固氮引起了很多兴趣。在此,我们研究了单分散双金属催化剂 Rh1Co3/CoO(011) 上 N2 到 NH3 热转化的催化机制,发现首选途径是类似于生物过程的缔合机制,其中交替氢化N2 发生,H2 在两个金属位点上都被激活。我们提出单分散的双金属 M1An 催化剂,其中掺杂的金属原子 M 取代了金属 A 氧化物表面上的氧原子,作为一种新的表面单簇催化剂(SCC)设计平台,用于仿生 N2-to- NH3 热转化。M1An催化剂的催化能力归因于掺杂金属M的电荷缓冲能力和协同金属A在催化中的互补作用。我们的工作为进一步优化 M1An 催化剂提供了见解和指导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号