当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convenient Reductive Methylation of Amines with Carbonates at Room Temperature
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-10-09 , DOI: 10.1002/chem.201502917 Yuehui Li , Iván Sorribes , Cristian Vicent , Kathrin Junge , Matthias Beller
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-10-09 , DOI: 10.1002/chem.201502917 Yuehui Li , Iván Sorribes , Cristian Vicent , Kathrin Junge , Matthias Beller
|
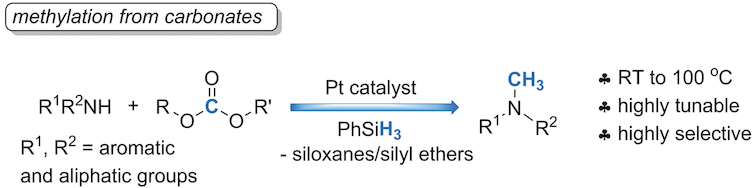
Methylation of amines is a fundamental and commonly used reaction in organic synthesis. Many methods are known including various reductive methylations using formaldehyde, formic acid, or carbon dioxide in the presence of reductants. However, several of these methods suffer from limited substrate scope and chemoselectivity because of the different nucleophilicities of substrates. In this respect, the combination of carbonates and hydrosilanes is a valuable methylation source in the presence of Pt‐based catalysts. This highly tunable method allows for methylation of both aromatic and aliphatic amines, and chemoselective methylation of aminoalcohols and diamines. Notably, the in situ‐formed catalyst can also be used for the reduction of carbonates to methanol at room temperature. Mechanistic insights on intermediates formed during the reaction pathway were obtained by using ESI mass spectrometry.
中文翻译:

室温下胺类与碳酸盐的便捷还原甲基化
胺的甲基化是有机合成中基本且常用的反应。已知许多方法,包括在还原剂存在下使用甲醛,甲酸或二氧化碳进行的各种还原甲基化。然而,由于底物的亲核性不同,这些方法中的几种受底物范围和化学选择性的限制。在这方面,碳酸盐和氢硅烷的组合是铂基催化剂存在下有价值的甲基化来源。这种高度可调的方法可以同时使芳香族和脂肪族胺甲基化,并实现氨基醇和二胺的化学选择性甲基化。值得注意的是,原位形成的催化剂也可用于在室温下将碳酸盐还原为甲醇。
更新日期:2015-10-09
中文翻译:

室温下胺类与碳酸盐的便捷还原甲基化
胺的甲基化是有机合成中基本且常用的反应。已知许多方法,包括在还原剂存在下使用甲醛,甲酸或二氧化碳进行的各种还原甲基化。然而,由于底物的亲核性不同,这些方法中的几种受底物范围和化学选择性的限制。在这方面,碳酸盐和氢硅烷的组合是铂基催化剂存在下有价值的甲基化来源。这种高度可调的方法可以同时使芳香族和脂肪族胺甲基化,并实现氨基醇和二胺的化学选择性甲基化。值得注意的是,原位形成的催化剂也可用于在室温下将碳酸盐还原为甲醇。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号