Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-synuclein Induces Mitochondrial Dysfunction through Spectrin and the Actin Cytoskeleton.
Neuron ( IF 14.7 ) Pub Date : 2018-Jan-03 , DOI: 10.1016/j.neuron.2017.11.036 Dalila G. Ordonez , Michael K. Lee , Mel B. Feany
Neuron ( IF 14.7 ) Pub Date : 2018-Jan-03 , DOI: 10.1016/j.neuron.2017.11.036 Dalila G. Ordonez , Michael K. Lee , Mel B. Feany
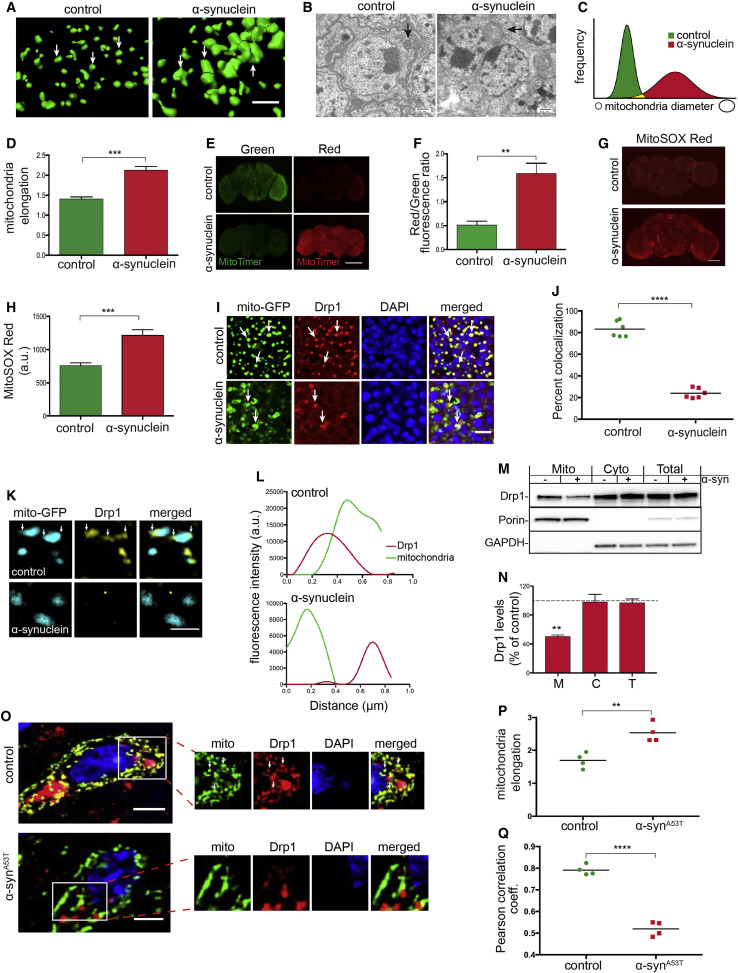
|
Genetics and neuropathology strongly link α-synuclein aggregation and neurotoxicity to the pathogenesis of Parkinson's disease and related α-synucleinopathies. Here we describe a new Drosophila model of α-synucleinopathy based on widespread expression of wild-type human α-synuclein, which shows robust neurodegeneration, early-onset locomotor deficits, and abundant α-synuclein aggregation. We use results of forward genetic screening and genetic analysis in our new model to demonstrate that α-synuclein expression promotes reorganization of the actin filament network and consequent mitochondrial dysfunction through altered Drp1 localization. Similar changes are present in a mouse α-synucleinopathy model and in postmortem brain tissue from patients with α-synucleinopathy. Importantly, we provide evidence that the interaction of α-synuclein with spectrin initiates pathological alteration of the actin cytoskeleton and downstream neurotoxicity. These findings suggest new therapeutic approaches for α-synuclein induced neurodegeneration.
中文翻译:

α-突触核蛋白通过血影蛋白和肌动蛋白细胞骨架诱导线粒体功能障碍。
遗传学和神经病理学将α-突触核蛋白的聚集和神经毒性与帕金森氏病的发病机理及相关的α-突触核蛋白致病联系紧密。在这里,我们基于野生型人α-突触核蛋白的广泛表达,描述了一种新的果蝇α-突触核蛋白病模型,该模型表现出强健的神经变性,早期发作的运动功能障碍和丰富的α-突触核蛋白聚集。我们在我们的新模型中使用正向遗传筛选和遗传分析的结果来证明,α-突触核蛋白表达通过改变的Drp1定位促进肌动蛋白丝网络的重组和随之而来的线粒体功能障碍。在小鼠α-突触核蛋白病模型和患有α-突触核蛋白病的患者的死后脑组织中也存在类似的变化。重要的,我们提供的证据表明,α-突触核蛋白与血影蛋白的相互作用会引发肌动蛋白细胞骨架的病理改变和下游神经毒性。这些发现提示了α-突触核蛋白诱导的神经变性的新治疗方法。
更新日期:2017-12-15
中文翻译:

α-突触核蛋白通过血影蛋白和肌动蛋白细胞骨架诱导线粒体功能障碍。
遗传学和神经病理学将α-突触核蛋白的聚集和神经毒性与帕金森氏病的发病机理及相关的α-突触核蛋白致病联系紧密。在这里,我们基于野生型人α-突触核蛋白的广泛表达,描述了一种新的果蝇α-突触核蛋白病模型,该模型表现出强健的神经变性,早期发作的运动功能障碍和丰富的α-突触核蛋白聚集。我们在我们的新模型中使用正向遗传筛选和遗传分析的结果来证明,α-突触核蛋白表达通过改变的Drp1定位促进肌动蛋白丝网络的重组和随之而来的线粒体功能障碍。在小鼠α-突触核蛋白病模型和患有α-突触核蛋白病的患者的死后脑组织中也存在类似的变化。重要的,我们提供的证据表明,α-突触核蛋白与血影蛋白的相互作用会引发肌动蛋白细胞骨架的病理改变和下游神经毒性。这些发现提示了α-突触核蛋白诱导的神经变性的新治疗方法。













































 京公网安备 11010802027423号
京公网安备 11010802027423号