Current Opinion in Colloid & Interface Science ( IF 7.9 ) Pub Date : 2017-10-03 , DOI: 10.1016/j.cocis.2017.09.005 Adriana P. Gerola , Paulo F.A. Costa , Faruk Nome , Frank Quina
|
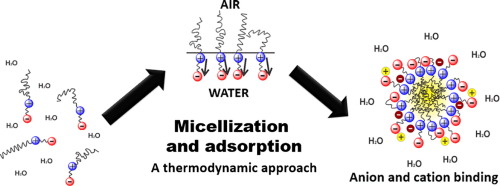
Zwitterionic surfactants are formally neutral but with headgroups containing both a positive charge center and a negative charge center separated from each other by a spacer group, with a long hydrophobic tail attached to one of the charge centers, usually but not always the positive charge center. The micellization and adsorption properties of zwitterionic surfactants depend on specifics of the surfactant structure such as the length m of the hydrophobic alkyl chain, the length n of the intercharge spacer and the nature of the headgroup charge centers. Micellization is favored by an increase in the hydrophobic tail length m, but goes through a maximum for interchange spacings of n = 3–4 methylene groups. There are additional effects from the presence of additional hydrophilic substituent groups in the spacer. Specific binding of anions and the cation valence of added electrolyte are factors that also modulate the micellization and adsorption properties of zwitterionic surfactants in the presence of added electrolyte. Anions in particular bind preferentially to zwitterionic micelles independent of the relative order of the charge centers in the headgroup. The anion binding affinities follow a Hofmeister series and impart a net negative charge to the micelles. Micellization is temperature-dependent and exhibits enthalpy-entropy compensation, with entropy dominant at lower temperatures and enthalpy more important at higher temperatures. The judicious manipulation of these factors permits control of the interfacial properties of zwitterionic surfactants, responsible for a wide range of applications in chromatography, electrophoresis, cloud point extraction, solubilization, stabilization of biomolecules and nanomaterials and catalysis.
中文翻译:

两性离子表面活性剂在空气/水界面的胶束化和吸附
两性离子表面活性剂在形式上是中性的,但头基包含一个正电荷中心和一个负电荷中心,它们之间通过一个间隔基团隔开,并且长疏水尾连接在一个电荷中心上,通常但不总是正电荷中心。两性离子表面活性剂的胶束化和吸附性能取决于表面活性剂结构的特性,例如疏水性烷基链的长度m,中间电荷间隔基的长度n和头基电荷中心的性质。疏水化尾部长度m的增加有利于胶束的形成,但对于n = 3-4个亚甲基。间隔基中存在额外的亲水取代基会产生其他影响。阴离子的特异性结合和添加的电解质的阳离子化合价也是在添加电解质的存在下还调节两性离子表面活性剂的胶束化和吸附性能的因素。阴离子特别优先与两性离子胶束结合,而与头基中电荷中心的相对顺序无关。阴离子结合亲和力遵循Hofmeister系列,并赋予胶束净负电荷。胶束化是温度依赖性的,并且表现出焓-熵补偿,在较低温度下熵占优势,而在较高温度下焓更重要。





























 京公网安备 11010802027423号
京公网安备 11010802027423号