当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Synthetic Routes to Triazolo-benzodiazepine Analogues: Expanding the Scope of the Bump-and-Hole Approach for Selective Bromo and Extra-Terminal (BET) Bromodomain Inhibition.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2015-10-01 , DOI: 10.1021/acs.jmedchem.5b01135 Matthias G J Baud 1, 2 , Enrique Lin-Shiao 1, 2 , Michael Zengerle 1 , Cynthia Tallant 2 , Alessio Ciulli 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2015-10-01 , DOI: 10.1021/acs.jmedchem.5b01135 Matthias G J Baud 1, 2 , Enrique Lin-Shiao 1, 2 , Michael Zengerle 1 , Cynthia Tallant 2 , Alessio Ciulli 1, 2
Affiliation
|
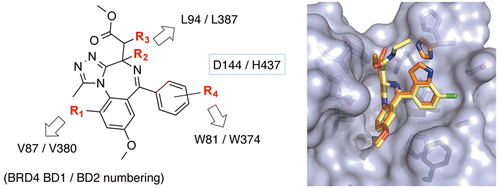
We describe new synthetic routes developed toward a range of substituted analogues of bromo and extra-terminal (BET) bromodomain inhibitors I-BET762/JQ1 based on the triazolo-benzodiazepine scaffold. These new routes allow for the derivatization of the methoxyphenyl and chlorophenyl rings, in addition to the diazepine ternary center and the side chain methylene moiety. Substitution at the level of the side chain methylene afforded compounds targeting specifically and potently engineered BET bromodomains designed as part of a bump and hole approach. We further demonstrate that marked selectivity for the second over the first bromodomain can be achieved with an indole derivative that exploits differential interaction with an aspartate/histidine conservative substitution on the BC loop of BET bromodomains.
中文翻译:

三唑并苯二氮卓类似物的新合成途径:扩大选择性溴和末端外 (BET) 溴域抑制的凹凸法的范围。
我们描述了基于三唑并苯二氮卓支架的一系列溴和末端外 (BET) 溴结构域抑制剂 I-BET762/JQ1 的取代类似物开发的新合成路线。除了二氮杂三元中心和侧链亚甲基部分之外,这些新路线还允许衍生甲氧基苯基和氯苯基环。侧链亚甲基水平的取代提供了针对特定和有效设计的 BET 溴结构域的化合物,该结构设计为凹凸方法的一部分。我们进一步证明,第二个对第一个溴结构域的显着选择性可以通过利用与 BET 溴结构域的 BC 环上的天冬氨酸/组氨酸保守取代的差异相互作用的吲哚衍生物来实现。
更新日期:2015-10-01
中文翻译:

三唑并苯二氮卓类似物的新合成途径:扩大选择性溴和末端外 (BET) 溴域抑制的凹凸法的范围。
我们描述了基于三唑并苯二氮卓支架的一系列溴和末端外 (BET) 溴结构域抑制剂 I-BET762/JQ1 的取代类似物开发的新合成路线。除了二氮杂三元中心和侧链亚甲基部分之外,这些新路线还允许衍生甲氧基苯基和氯苯基环。侧链亚甲基水平的取代提供了针对特定和有效设计的 BET 溴结构域的化合物,该结构设计为凹凸方法的一部分。我们进一步证明,第二个对第一个溴结构域的显着选择性可以通过利用与 BET 溴结构域的 BC 环上的天冬氨酸/组氨酸保守取代的差异相互作用的吲哚衍生物来实现。

































 京公网安备 11010802027423号
京公网安备 11010802027423号