Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2017-10-07 , DOI: 10.1016/j.abb.2017.09.019 Zhiqiang Duan , Jiafu Zhao , Houqiang Xu , Haixu Xu , Xinqin Ji , Xiang Chen , Jianming Xiong
|
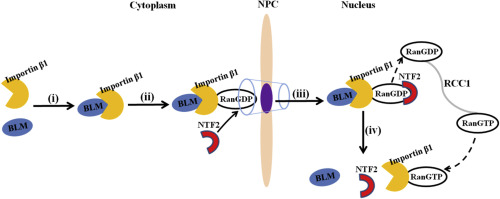
Numerous studies have shown that nuclear localization of BLM protein, a member of the RecQ helicases, mediated by nuclear localization signal (NLS) is critical for DNA recombination, replication and transcription, but the mechanism by which BLM protein is imported into the nucleus remains unknown. In this study, the nuclear import pathway for BLM was investigated. We found that nuclear import of BLM was inhibited by two dominant-negative mutants of importin β1 and NTF2/E42K, which lacks the ability to bind Ran and RanGDP, respectively, but was not inhibited by the Ran/Q69L, which is deficient in GTP hydrolysis. Further studies revealed that nuclear import of BLM was reconstituted using importin β1, RanGDP and NTF2 in digitonin-permeabilized HeLa cells. Moreover, BLM had direct binding to importin β1 through its NLS domain with the 14–16 HEAT repeats of importin β1. Furthermore, importin β1, Ran or NTF2 depletion by siRNA disrupted the accumulation of BLM protein in the nucleus. These results showed that BLM enters the nucleus via the importin β1, RanGDP and NTF2 dependent pathway, demonstrating for the first time the nuclear trafficking mechanism of a DNA helicase.
中文翻译:

BLM蛋白的核输入途径的表征
大量研究表明,RecQ解旋酶成员BLM蛋白的核定位是由核定位信号(NLS)介导的,对于DNA重组,复制和转录至关重要,但是将BLM蛋白导入细胞核的机制仍然未知。在这项研究中,BLM的核进口途径进行了调查。我们发现,BLM的核输入被importinβ1和NTF2 / E42K的两个显性负突变抑制,它们分别缺乏结合Ran和RanGDP的能力,但未被Ran / Q69L抑制,后者缺乏GTP。水解。进一步的研究表明,在洋地黄素透化的HeLa细胞中,使用importinβ1,RanGDP和NTF2重建了BLM的核输入。而且,BLM通过其NLS结构域与importinβ1的14–16 HEAT重复序列直接结合importinβ1。此外,siRNA消耗了importinβ1,Ran或NTF2,破坏了BLM蛋白在细胞核中的积累。这些结果表明,BLM通过importinβ1,RanGDP和NTF2依赖性途径进入细胞核,首次证明了DNA解旋酶的核转运机制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号