Insect Biochemistry and Molecular Biology ( IF 3.2 ) Pub Date : 2017-12-02 , DOI: 10.1016/j.ibmb.2017.11.009 Lianyun Lin , Chen Liu , Juan Qin , Jie Wang , Shengjie Dong , Wei Chen , Weiyi He , Qingzhi Gao , Minsheng You , Zhiguang Yuchi
|
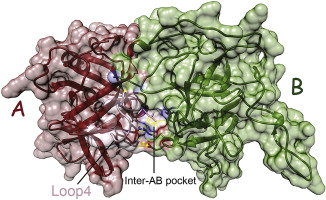
Ryanodine receptors (RyRs) are large calcium-release channels located in sarcoplasmic reticulum membrane. They play a central role in excitation-contraction coupling of muscle cells. Three commercialized insecticides targeting pest RyRs generate worldwide sales over 2 billion U.S. dollars annually, but the structure of insect RyRs remains elusive, hindering our understanding of the mode of action of RyR-targeting insecticides and the development of insecticide resistance in pests. Here we present the crystal structure of RyR N-terminal domain (NTD) (residue 1–205) at 2.84 Å resolution from the diamondback moth (DBM), Plutella xylostella, a destructive pest devouring cruciferous crops all over the world. Similar to its mammalian homolog, DBM RyR NTD consists of a beta-trefoil folding motif and a flanking alpha helix. Interestingly, two regions in NTD interacting with neighboring domains showed distinguished conformations in DBM relative to mammalian RyRs. Using homology modeling and molecular dynamics simulation, we created a structural model of the N-terminal three domains, showing two unique binding pockets that could be targeted by potential species-specific insecticides. Thermal melt experiment showed that the stability of DBM RyR NTD was higher than mammalian RyRs, probably due to a stable intra-domain disulfide bond observed in the crystal structure. Previously DBM NTD was shown to be one of the two critical regions to interact with insecticide flubendiamide, but isothermal titration calorimetry experiments negated DBM NTD alone as a major binding site for flubendiamide.
中文翻译:

小菜蛾Ryananodine受体N末端域的晶体结构揭示了两个潜在的物种特异性杀虫剂靶向位点
Ryanodine受体(RyRs)是位于肌质网膜中的大钙释放通道。它们在肌肉细胞的兴奋收缩耦合中起着核心作用。三种针对害虫RyRs的商业化杀虫剂每年在全球范围内的销售额超过20亿美元,但是昆虫RyRs的结构仍然难以捉摸,这阻碍了我们对针对RyR的杀虫剂的作用方式以及对害虫抗药性的了解。在这里,我们以小菜蛾(Plutella xylostella)小菜蛾(DBM)的2.84Å分辨率介绍了RyR N末端结构域(NTD)(残基1–205)的晶体结构,一种破坏性的害虫,在世界范围内吞噬十字花科作物。DBM RyR NTD与它的哺乳动物同系物相似,由一个β-三叶折叠基序和一个侧面的α螺旋组成。有趣的是,相对于哺乳动物RyRs,NTD中与相邻域相互作用的两个区域在DBM中显示出独特的构象。使用同源性建模和分子动力学模拟,我们创建了N末端三个域的结构模型,显示了两个独特的结合口袋,可能被潜在的物种特异性杀虫剂作为目标。热熔实验表明,DBM RyR NTD的稳定性高于哺乳动物RyRs,这可能是由于在晶体结构中观察到了稳定的域内二硫键。以前,DBM NTD被证明是与杀虫剂氟苯二酰胺相互作用的两个关键区域之一,




















































 京公网安备 11010802027423号
京公网安备 11010802027423号