当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New (3-(1H-benzo[d]imidazol-2-yl))/(3-(3H-imidazo[4,5-b]pyridin-2-yl))-(1H-indol-5-yl)(3,4,5-trimethoxyphenyl)methanone conjugates as tubulin polymerization inhibitors†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7md00450h Kishore Mullagiri 1 , V Lakshma Nayak 1 , Satish Sunkari 1 , Geeta Sai Mani 2 , Sravanthi Devi Guggilapu 2 , Burri Nagaraju 1 , Abdullah Alarifi 3 , Ahmed Kamal 1, 2, 3
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7md00450h Kishore Mullagiri 1 , V Lakshma Nayak 1 , Satish Sunkari 1 , Geeta Sai Mani 2 , Sravanthi Devi Guggilapu 2 , Burri Nagaraju 1 , Abdullah Alarifi 3 , Ahmed Kamal 1, 2, 3
Affiliation
|
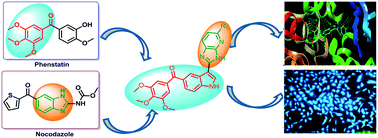
A series of new (3-(1H-benzo[d]imidazol-2-yl))/(3-(3H-imidazo[4,5-b]pyridin-2-yl))-(1H-indol-5-yl)(3,4,5-trimethoxyphenyl)methanone conjugates 4–6(a–i) were synthesized and evaluated for their antiproliferative activity on selected human cancer cell lines such as prostate (DU-145), lung (A549), cervical (HeLa) and breast (MCF-7). Most of these conjugates showed considerable cytotoxicity with IC50 values ranging from 0.54 to 31.86 μM. Among them, compounds 5g and 6f showed significant activity against human prostate cancer cell line DU-145 with IC50 values of 0.68 μM and 0.54 μM, respectively. Tubulin polymerization assay and immunofluorescence analysis results suggest that these compounds effectively inhibit microtubule assembly formation in DU-145. Further, the apoptosis-inducing ability of these derivatives (5g and 6f) was confirmed by Hoechst staining, measurement of mitochondrial membrane potential and ROS generation and annexin V-FITC assays.
中文翻译:

新的(3-(1H-苯并[d]咪唑-2-基))/(3-(3H-咪唑[4,5-b]吡啶-2-基))-(1H-吲哚-5-基) (3,4,5-三甲氧基苯基)甲酮缀合物作为微管蛋白聚合抑制剂†
一系列新的(3-( 1H-苯并[ d ]咪唑-2-基))/(3-( 3H-咪唑[4,5 - b ]吡啶-2-基))- ( 1H- indol-5-yl)(3,4,5-trimethoxyphenyl)methanone conjugates 4-6(a-i)被合成并评估它们对选定的人类癌细胞系如前列腺 (DU-145)、肺 ( A549)、宫颈 (HeLa) 和乳房 (MCF-7)。大多数这些缀合物显示出相当大的细胞毒性,IC 50值范围为 0.54 至 31.86 μM。其中,化合物5g和6f对人前列腺癌细胞系 DU-145 显示出显着的活性,IC 50值分别为 0.68 μM 和 0.54 μM。微管蛋白聚合测定和免疫荧光分析结果表明,这些化合物可有效抑制 DU-145 中微管组装的形成。此外,这些衍生物( 5g和6f )的细胞凋亡诱导能力通过 Hoechst 染色、线粒体膜电位和 ROS 生成的测量以及膜联蛋白 V-FITC 测定得到证实。
更新日期:2017-12-12
中文翻译:

新的(3-(1H-苯并[d]咪唑-2-基))/(3-(3H-咪唑[4,5-b]吡啶-2-基))-(1H-吲哚-5-基) (3,4,5-三甲氧基苯基)甲酮缀合物作为微管蛋白聚合抑制剂†
一系列新的(3-( 1H-苯并[ d ]咪唑-2-基))/(3-( 3H-咪唑[4,5 - b ]吡啶-2-基))- ( 1H- indol-5-yl)(3,4,5-trimethoxyphenyl)methanone conjugates 4-6(a-i)被合成并评估它们对选定的人类癌细胞系如前列腺 (DU-145)、肺 ( A549)、宫颈 (HeLa) 和乳房 (MCF-7)。大多数这些缀合物显示出相当大的细胞毒性,IC 50值范围为 0.54 至 31.86 μM。其中,化合物5g和6f对人前列腺癌细胞系 DU-145 显示出显着的活性,IC 50值分别为 0.68 μM 和 0.54 μM。微管蛋白聚合测定和免疫荧光分析结果表明,这些化合物可有效抑制 DU-145 中微管组装的形成。此外,这些衍生物( 5g和6f )的细胞凋亡诱导能力通过 Hoechst 染色、线粒体膜电位和 ROS 生成的测量以及膜联蛋白 V-FITC 测定得到证实。































 京公网安备 11010802027423号
京公网安备 11010802027423号