当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Activity in Lithium-Treated Core–Shell MoOx/MoS2 Nanowires
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-09-29 00:00:00 , DOI: 10.1021/acs.jpcc.5b05640 Dustin R. Cummins 1, 2 , Ulises Martinez 1 , Rajesh Kappera 1, 3 , Damien Voiry 3 , Alejandro Martinez-Garcia 2 , Jacek Jasinski 2 , Dan Kelly 4 , Manish Chhowalla 3 , Aditya D. Mohite 1 , Mahendra K. Sunkara 2 , Gautam Gupta 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-09-29 00:00:00 , DOI: 10.1021/acs.jpcc.5b05640 Dustin R. Cummins 1, 2 , Ulises Martinez 1 , Rajesh Kappera 1, 3 , Damien Voiry 3 , Alejandro Martinez-Garcia 2 , Jacek Jasinski 2 , Dan Kelly 4 , Manish Chhowalla 3 , Aditya D. Mohite 1 , Mahendra K. Sunkara 2 , Gautam Gupta 1
Affiliation
|
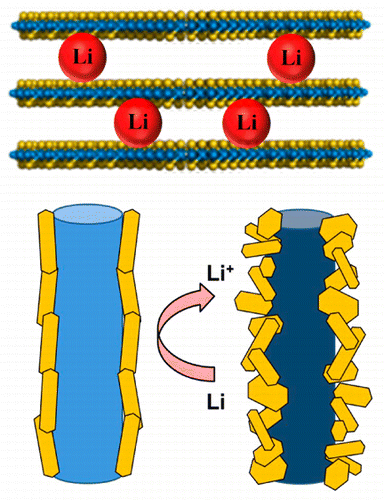
Significant interest has grown in the development of earth-abundant and efficient catalytic materials for hydrogen generation. Layered transition metal dichalcogenides present opportunities for efficient electrocatalytic systems. Here, we report the modification of 1D MoOx/MoS2 core–shell nanostructures by lithium intercalation and the corresponding changes in morphology, structure, and mechanism of H2 evolution. The 1D nanowires exhibit significant improvement in H2 evolution properties after lithiation, reducing the hydrogen evolution reaction (HER) onset potential by ∼50 mV and increasing the generated current density by ∼600%. The high electrochemical activity in the nanowires results from disruption of MoS2 layers in the outer shell, leading to increased activity and concentration of defect sites. This is in contrast to the typical mechanism of improved catalysis following lithium exfoliation, i.e., crystal phase transformation. These structural changes are verified by a combination of Raman and X-ray photoelectron spectroscopy (XPS).
中文翻译:

锂基核壳MoO x / MoS 2纳米线的催化活性
人们对开发用于地球资源的富含地球的高效催化材料的兴趣日益浓厚。层状过渡金属二卤化物为高效的电催化系统提供了机会。在这里,我们报道了通过锂嵌入对一维MoO x / MoS 2核-壳纳米结构的修饰以及H 2析出的形态,结构和机理的相应变化。一维纳米线在锂化后显示出H 2放出特性的显着改善,将氢放出反应(HER)的起始电势降低了约50 mV,并将产生的电流密度提高了约600%。纳米线中的高电化学活性来自MoS 2的破坏外壳中的金属层,导致活性增加和缺陷部位的集中。这与锂剥落后改善催化作用(即晶相转变)的典型机理相反。这些结构变化通过拉曼光谱和X射线光电子能谱(XPS)的组合得到验证。
更新日期:2015-09-29
中文翻译:

锂基核壳MoO x / MoS 2纳米线的催化活性
人们对开发用于地球资源的富含地球的高效催化材料的兴趣日益浓厚。层状过渡金属二卤化物为高效的电催化系统提供了机会。在这里,我们报道了通过锂嵌入对一维MoO x / MoS 2核-壳纳米结构的修饰以及H 2析出的形态,结构和机理的相应变化。一维纳米线在锂化后显示出H 2放出特性的显着改善,将氢放出反应(HER)的起始电势降低了约50 mV,并将产生的电流密度提高了约600%。纳米线中的高电化学活性来自MoS 2的破坏外壳中的金属层,导致活性增加和缺陷部位的集中。这与锂剥落后改善催化作用(即晶相转变)的典型机理相反。这些结构变化通过拉曼光谱和X射线光电子能谱(XPS)的组合得到验证。































 京公网安备 11010802027423号
京公网安备 11010802027423号