当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vibrational Stark Effects To Identify Ion Pairing and Determine Reduction Potentials in Electrolyte-Free Environments
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-01-17 , DOI: 10.1021/ja512302c Tomoyasu Mani 1 , David C. Grills 1 , John R. Miller 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-01-17 , DOI: 10.1021/ja512302c Tomoyasu Mani 1 , David C. Grills 1 , John R. Miller 1
Affiliation
|
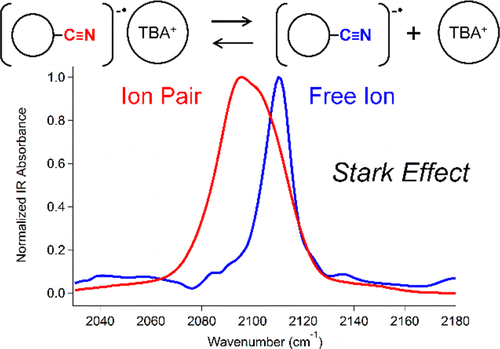
A recently developed instrument for time-resolved infrared detection following pulse radiolysis has been used to measure the ν(C≡N) IR band of the radical anion of a CN-substituted fluorene in tetrahydrofuran. Specific vibrational frequencies can exhibit distinct frequency shifts due to ion pairing, which can be explained in the framework of the vibrational Stark effect. Measurements of the ratio of free ions and ion pairs in different electrolyte concentrations allowed us to obtain an association constant and free energy change for ion pairing. This new method has the potential to probe the geometry of ion pairing and allows the reduction potentials of molecules to be determined in the absence of electrolyte in an environment of low dielectric constant.
中文翻译:

在无电解质环境中识别离子对和确定还原电位的振动斯塔克效应
最近开发的用于脉冲辐射分解后时间分辨红外检测的仪器已用于测量四氢呋喃中 CN 取代芴的自由基阴离子的 ν(C≡N) 红外波段。由于离子配对,特定的振动频率可以表现出明显的频移,这可以在振动斯塔克效应的框架中解释。测量不同电解质浓度下自由离子和离子对的比率,使我们能够获得离子对的缔合常数和自由能变化。这种新方法有可能探测离子对的几何形状,并允许在低介电常数环境中没有电解质的情况下确定分子的还原电位。
更新日期:2015-01-17
中文翻译:

在无电解质环境中识别离子对和确定还原电位的振动斯塔克效应
最近开发的用于脉冲辐射分解后时间分辨红外检测的仪器已用于测量四氢呋喃中 CN 取代芴的自由基阴离子的 ν(C≡N) 红外波段。由于离子配对,特定的振动频率可以表现出明显的频移,这可以在振动斯塔克效应的框架中解释。测量不同电解质浓度下自由离子和离子对的比率,使我们能够获得离子对的缔合常数和自由能变化。这种新方法有可能探测离子对的几何形状,并允许在低介电常数环境中没有电解质的情况下确定分子的还原电位。












































 京公网安备 11010802027423号
京公网安备 11010802027423号