当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Depolymerization of Oxidized Lignin Catalyzed by Formic Acid Exploits an Unconventional Elimination Mechanism Involving 3c–4e Bonding: A DFT Mechanistic Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-09-30 00:00:00 , DOI: 10.1021/acscatal.5b01095
Shuanglin Qu 1 , Yanfeng Dang 1 , Chunyu Song 1 , Jiandong Guo 1 , Zhi-Xiang Wang 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-09-30 00:00:00 , DOI: 10.1021/acscatal.5b01095
Shuanglin Qu 1 , Yanfeng Dang 1 , Chunyu Song 1 , Jiandong Guo 1 , Zhi-Xiang Wang 1, 2
Affiliation
|
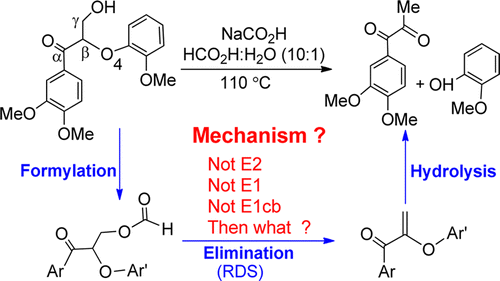
A DFT study has been performed to gain insight into the formic-acid-catalyzed depolymerization of the oxidized lignin model (1ox) to monoaromatics, developed by Stahl et al. (Nature2014, 515, 249–252). The conversion proceeds sequentially via formylation, elimination, and hydrolysis. Intriguingly, the elimination process exploits an unconventional mechanism different from the known ones such as E2 and E1cb. The new mechanism is characterized by passing through an intermediate stabilized by a proton-shared 3c–4e bond (HCOO⊖···H⊕···⊖O═Cα) and by shifting the 3c–4e bond to the 3c–4e HCOO⊖···H⊕···⊖OOCH bond in the joint leaving group that is originally a regular H-bond (HCOO–H···OOCH−). According to these characteristics, as well as the important role of the original HCOO–H···OOCH– bond, we term the mechanism as E1H-3c4e elimination. The root-cause of the E1H-3c4e elimination is that the poor leaving formate group is less competitive in stabilizing the negative charge resulted from Hβ abstraction by the HCOO– base than the nearby carbonyl group (Cα═O) that can utilize the negative charge to form a stabilizing 3c–4e bond with a formic acid molecule. In addition, the study characterizes versatile roles of formic acid in achieving the whole transformation, which accounts for why the HCO2H/NaCO2H medium works so elegantly for 1ox depolymerizaion.
中文翻译:

甲酸催化氧化木质素的解聚利用涉及3c–4e键的非常规消除机理:DFT机理研究
已经进行了DFT研究,以了解由氧化硫酸的木质素模型(1 ox)转化为单芳烃的甲酸催化解聚反应,该反应由Stahl等人开发。(《自然》2014年第515期249–252页)。转化通过甲酰化,消除和水解顺序进行。有趣的是,消除过程利用了一种不同于常规机制的已知机制,例如E2和E1cb。新机制的特征在于,通过由一个质子共享3C-4E键稳定的中间体(HCOO ⊖ ...ħ ⊕ ... ⊖ O = C α)和由图3c-4E键转移到图3c-4E HCOO ⊖···ħ ⊕ ... ⊖ OOCH联合离去基团中键即原本是常规的H-键(HCOO-H···OOCH-)。根据这些特征以及原始HCOO–H··OOCH–键的重要作用,我们将该机理称为E1H-3c4e消除。所述E1H-3c4e消除的根本原因是贫困离去甲酸基团是稳定的负电荷导致选自H缺乏竞争力β由HCOO抽象-基比附近羰基(C α = O),其可以利用负电荷与甲酸分子形成稳定的3c–4e键。此外,该研究还表征了甲酸在实现整个转化过程中的多种作用,这也解释了为什么HCO2 H / NaCO 2 H介质非常适合1氧解聚反应。
更新日期:2015-09-30
中文翻译:

甲酸催化氧化木质素的解聚利用涉及3c–4e键的非常规消除机理:DFT机理研究
已经进行了DFT研究,以了解由氧化硫酸的木质素模型(1 ox)转化为单芳烃的甲酸催化解聚反应,该反应由Stahl等人开发。(《自然》2014年第515期249–252页)。转化通过甲酰化,消除和水解顺序进行。有趣的是,消除过程利用了一种不同于常规机制的已知机制,例如E2和E1cb。新机制的特征在于,通过由一个质子共享3C-4E键稳定的中间体(HCOO ⊖ ...ħ ⊕ ... ⊖ O = C α)和由图3c-4E键转移到图3c-4E HCOO ⊖···ħ ⊕ ... ⊖ OOCH联合离去基团中键即原本是常规的H-键(HCOO-H···OOCH-)。根据这些特征以及原始HCOO–H··OOCH–键的重要作用,我们将该机理称为E1H-3c4e消除。所述E1H-3c4e消除的根本原因是贫困离去甲酸基团是稳定的负电荷导致选自H缺乏竞争力β由HCOO抽象-基比附近羰基(C α = O),其可以利用负电荷与甲酸分子形成稳定的3c–4e键。此外,该研究还表征了甲酸在实现整个转化过程中的多种作用,这也解释了为什么HCO2 H / NaCO 2 H介质非常适合1氧解聚反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号