当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Minimizing Aryloxy Elimination in RhI‐Catalyzed Asymmetric Hydrogenation of β‐Aryloxyacrylic Acids using a Mixed‐Ligand Strategy
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-10-01 , DOI: 10.1002/chem.201503229 Yang Li , Zheng Wang , Kuiling Ding
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-10-01 , DOI: 10.1002/chem.201503229 Yang Li , Zheng Wang , Kuiling Ding
|
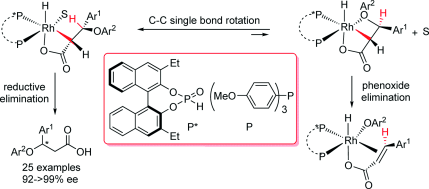
The first example of efficient asymmetric hydrogenation of challenging β‐aryloxyacrylic acids was realized using a RhI‐complex based on the heterocombination of a readily available chiral monodentate secondary phosphine oxide (SPO) and an achiral monodentate phosphine ligand as the catalyst. Excellent enantioselectivities (92–>99 % ee) were achieved for a wide variety of chiral β‐aryloxypropionic acids with minor aryloxy elimination in most cases. The resultant products were readily transformed into biologically active compounds through simple synthetic manipulations.
中文翻译:

使用混合配体策略最大限度地减少RhI催化β-芳氧基丙烯酸不对称加氢中的芳氧基消除
使用基于容易获得的手性单齿仲氧化膦(SPO)和非手性单齿膦配体作为催化剂的Rh I-络合物,实现了具有挑战性的β-芳氧基丙烯酸高效不对称氢化的第一个例子。在大多数情况下,对多种手性β-芳氧基丙酸具有优异的对映选择性(92-> 99%ee),而芳氧基的消除则很少。通过简单的合成操作,容易将所得产物转化为生物活性化合物。
更新日期:2015-10-01
中文翻译:

使用混合配体策略最大限度地减少RhI催化β-芳氧基丙烯酸不对称加氢中的芳氧基消除
使用基于容易获得的手性单齿仲氧化膦(SPO)和非手性单齿膦配体作为催化剂的Rh I-络合物,实现了具有挑战性的β-芳氧基丙烯酸高效不对称氢化的第一个例子。在大多数情况下,对多种手性β-芳氧基丙酸具有优异的对映选择性(92-> 99%ee),而芳氧基的消除则很少。通过简单的合成操作,容易将所得产物转化为生物活性化合物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号