当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AnIn SituDirecting Group Strategy for Chiral Anion Phase-Transfer Fluorination of Allylic Alcohols
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-09-09 , DOI: 10.1021/ja507468u Weiwei Zi 1 , Yi-Ming Wang , F Dean Toste
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-09-09 , DOI: 10.1021/ja507468u Weiwei Zi 1 , Yi-Ming Wang , F Dean Toste
Affiliation
|
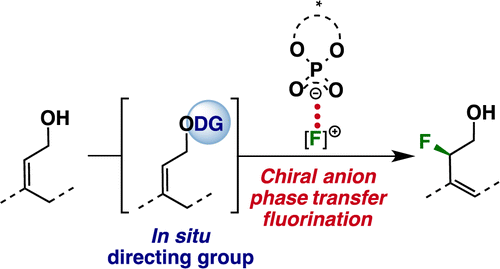
An enantioselective fluorination of allylic alcohols under chiral anion phase-transfer conditions is reported. The in situ generation of a directing group proved crucial for achieving effective enantiocontrol. In the presence of such a directing group, a range of acyclic substrates underwent fluorination to afford highly enantioenriched α-fluoro homoallylic alcohols. Mechanistic studies suggest that this transformation proceeds through a concerted enantiodetermining transition state involving both C–F bond formation and C–H bond cleavage.
中文翻译:

烯丙醇手性阴离子相转移氟化的原位指导组策略
报道了在手性阴离子相转移条件下烯丙醇的对映选择性氟化。事实证明,原位生成导向基团对于实现有效的对映体控制至关重要。在这种导向基团的存在下,一系列无环底物发生氟化,得到高度对映体富集的α-氟高烯丙醇。机理研究表明,这种转变通过协调的对映决定过渡态进行,涉及 C-F 键形成和 C-H 键裂解。
更新日期:2014-09-09
中文翻译:

烯丙醇手性阴离子相转移氟化的原位指导组策略
报道了在手性阴离子相转移条件下烯丙醇的对映选择性氟化。事实证明,原位生成导向基团对于实现有效的对映体控制至关重要。在这种导向基团的存在下,一系列无环底物发生氟化,得到高度对映体富集的α-氟高烯丙醇。机理研究表明,这种转变通过协调的对映决定过渡态进行,涉及 C-F 键形成和 C-H 键裂解。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号