当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
M2(m-dobdc) (M = Mg, Mn, Fe, Co, Ni) Metal–Organic Frameworks Exhibiting Increased Charge Density and Enhanced H2Binding at the Open Metal Sites
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-08-18 , DOI: 10.1021/ja506230r Matthew T. Kapelewski 1 , Stephen J. Geier 1 , Matthew R. Hudson 2 , David Stück 1 , Jarad A. Mason 1 , Jocienne N. Nelson 3 , Dianne J. Xiao 1 , Zeric Hulvey 2, 4 , Elizabeth Gilmour 3 , Stephen A. FitzGerald 3 , Martin Head-Gordon 1 , Craig M. Brown 2, 5 , Jeffrey R. Long 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-08-18 , DOI: 10.1021/ja506230r Matthew T. Kapelewski 1 , Stephen J. Geier 1 , Matthew R. Hudson 2 , David Stück 1 , Jarad A. Mason 1 , Jocienne N. Nelson 3 , Dianne J. Xiao 1 , Zeric Hulvey 2, 4 , Elizabeth Gilmour 3 , Stephen A. FitzGerald 3 , Martin Head-Gordon 1 , Craig M. Brown 2, 5 , Jeffrey R. Long 1
Affiliation
|
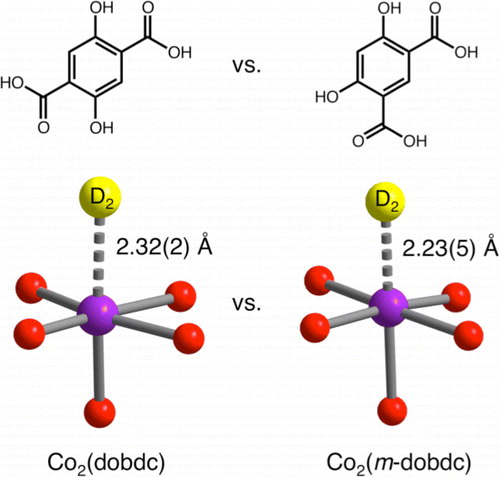
The well-known frameworks of the type M2(dobdc) (dobdc(4-) = 2,5-dioxido-1,4-benzenedicarboxylate) have numerous potential applications in gas storage and separations, owing to their exceptionally high concentration of coordinatively unsaturated metal surface sites, which can interact strongly with small gas molecules such as H2. Employing a related meta-functionalized linker that is readily obtained from resorcinol, we now report a family of structural isomers of this framework, M2(m-dobdc) (M = Mg, Mn, Fe, Co, Ni; m-dobdc(4-) = 4,6-dioxido-1,3-benzenedicarboxylate), featuring exposed M(2+) cation sites with a higher apparent charge density. The regioisomeric linker alters the symmetry of the ligand field at the metal sites, leading to increases of 0.4-1.5 kJ/mol in the H2 binding enthalpies relative to M2(dobdc). A variety of techniques, including powder X-ray and neutron diffraction, inelastic neutron scattering, infrared spectroscopy, and first-principles electronic structure calculations, are applied in elucidating how these subtle structural and electronic differences give rise to such increases. Importantly, similar enhancements can be anticipated for the gas storage and separation properties of this new family of robust and potentially inexpensive metal-organic frameworks.
中文翻译:

M2(m-dobdc)(M = Mg、Mn、Fe、Co、Ni)金属-有机骨架在开放金属位点表现出增加的电荷密度和增强的 H2 结合
众所周知的 M2(dobdc) 型(dobdc(4-) = 2,5-dioxido-1,4-benzodicarboxylate)在气体储存和分离中具有许多潜在的应用,因为它们具有极高浓度的配位不饱和化合物金属表面位点,可与小气体分子(如 H2)发生强烈相互作用。利用易于从间苯二酚中获得的相关元功能化接头,我们现在报告了该框架的一系列结构异构体,M2(m-dobdc)(M = Mg、Mn、Fe、Co、Ni;m-dobdc(4 -) = 4,6-二氧化-1,3-苯二甲酸酯),具有暴露的 M(2+) 阳离子位点,具有更高的表观电荷密度。区域异构接头改变了金属位点配体场的对称性,导致 H2 结合焓相对于 M2(dobdc) 增加 0.4-1.5 kJ/mol。多种技法,包括粉末 X 射线和中子衍射、非弹性中子散射、红外光谱和第一性原理电子结构计算在内,用于阐明这些细微的结构和电子差异如何导致这种增加。重要的是,可以预期这种新的坚固且可能廉价的金属有机骨架系列的气体存储和分离性能将得到类似的增强。
更新日期:2014-08-18
中文翻译:

M2(m-dobdc)(M = Mg、Mn、Fe、Co、Ni)金属-有机骨架在开放金属位点表现出增加的电荷密度和增强的 H2 结合
众所周知的 M2(dobdc) 型(dobdc(4-) = 2,5-dioxido-1,4-benzodicarboxylate)在气体储存和分离中具有许多潜在的应用,因为它们具有极高浓度的配位不饱和化合物金属表面位点,可与小气体分子(如 H2)发生强烈相互作用。利用易于从间苯二酚中获得的相关元功能化接头,我们现在报告了该框架的一系列结构异构体,M2(m-dobdc)(M = Mg、Mn、Fe、Co、Ni;m-dobdc(4 -) = 4,6-二氧化-1,3-苯二甲酸酯),具有暴露的 M(2+) 阳离子位点,具有更高的表观电荷密度。区域异构接头改变了金属位点配体场的对称性,导致 H2 结合焓相对于 M2(dobdc) 增加 0.4-1.5 kJ/mol。多种技法,包括粉末 X 射线和中子衍射、非弹性中子散射、红外光谱和第一性原理电子结构计算在内,用于阐明这些细微的结构和电子差异如何导致这种增加。重要的是,可以预期这种新的坚固且可能廉价的金属有机骨架系列的气体存储和分离性能将得到类似的增强。








































 京公网安备 11010802027423号
京公网安备 11010802027423号