当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopic Signature for Stable β-Amyloid Fibrils versus β-Sheet-Rich Oligomers
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acs.jpcb.7b10765 Justin P. Lomont 1 , Kacie L. Rich 1 , Michał Maj 1 , Jia-Jung Ho 1 , Joshua S. Ostrander 1 , Martin T. Zanni 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acs.jpcb.7b10765 Justin P. Lomont 1 , Kacie L. Rich 1 , Michał Maj 1 , Jia-Jung Ho 1 , Joshua S. Ostrander 1 , Martin T. Zanni 1
Affiliation
|
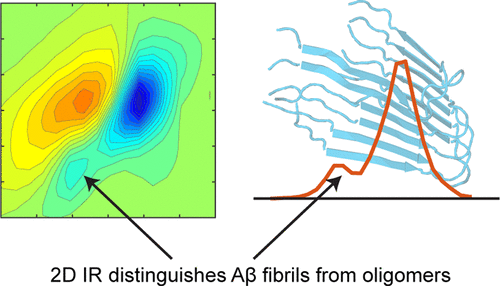
We use two-dimensional IR (2D IR) spectroscopy to explore fibril formation for the two predominant isoforms of the β-amyloid (Aβ1-40 and Aβ1-42) protein associated with Alzheimer’s disease. Two-dimensional IR spectra resolve a transition at 1610 cm–1 in Aβ fibrils that does not appear in other Aβ aggregates, even those with predominantly β-sheet-structure-like oligomers. This transition is not resolved in linear IR spectroscopy because it lies under the broad band centered at 1625 cm–1, which is the traditional infrared signature for amyloid fibrils. The feature is prominent in 2D IR spectra because 2D lineshapes are narrower and scale nonlinearly with transition dipole strengths. Transmission electron microscopy measurements demonstrate that the 1610 cm–1 band is a positive identification of amyloid fibrils. Sodium dodecyl sulfate micelles that solubilize and disaggregate preaggregated Aβ samples deplete the 1625 cm–1 band but do not affect the 1610 cm–1 band, demonstrating that the 1610 cm–1 band is due to very stable fibrils. We demonstrate that the 1610 cm–1 transition arises from amide I modes by mutating out the only side-chain residue that could give rise to this transition, and we explore the potential structural origins of the transition by simulating 2D IR spectra based on Aβ crystal structures. It was not previously possible to distinguish stable Aβ fibrils from the less stable β-sheet-rich oligomers with infrared light. This 2D IR signature will be useful for Alzheimer’s research on Aβ aggregation, fibril formation, and toxicity.
中文翻译:

稳定的β-淀粉样蛋白原纤维与富含β-Sheet的寡聚体的光谱特征
我们使用二维红外(2D IR)光谱研究与阿尔茨海默氏病相关的β-淀粉样蛋白(Aβ1-40和Aβ1-42)蛋白的两个主要同工型的原纤维形成。二维红外光谱解析了Aβ原纤维在1610 cm –1处的过渡,该过渡在其他Aβ聚集体中不出现,即使那些主要具有β-片层结构样的低聚物也是如此。线性红外光谱无法解决此过渡问题,因为它位于以1625 cm –1为中心的宽带下,这是淀粉样蛋白纤维的传统红外特征。该特征在2D红外光谱中很显着,因为2D线形更窄,并且随着跃迁偶极子强度而非线性缩放。透射电子显微镜测量表明,1610 cm –1谱带是淀粉样蛋白原纤维的阳性鉴定。溶解和分解预聚集的Aβ样品的十二烷基硫酸钠胶束消耗1625 cm –1谱带,但不影响1610 cm –1谱带,表明1610 cm –1谱带是由于非常稳定的原纤维。我们证明了1610厘米–1过渡态是由酰胺I模式产生的,它是通过突变掉唯一可能引起该过渡的侧链残基而产生的,我们通过模拟基于Aβ晶体结构的2D红外光谱,探索了该过渡态的潜在结构起源。以前不可能用红外线将稳定的Aβ原纤维与较不稳定的富含β-折叠的低聚物区分开来。该2D IR签名将对阿尔茨海默氏症对Aβ聚集,原纤维形成和毒性的研究有用。
更新日期:2017-12-27
中文翻译:

稳定的β-淀粉样蛋白原纤维与富含β-Sheet的寡聚体的光谱特征
我们使用二维红外(2D IR)光谱研究与阿尔茨海默氏病相关的β-淀粉样蛋白(Aβ1-40和Aβ1-42)蛋白的两个主要同工型的原纤维形成。二维红外光谱解析了Aβ原纤维在1610 cm –1处的过渡,该过渡在其他Aβ聚集体中不出现,即使那些主要具有β-片层结构样的低聚物也是如此。线性红外光谱无法解决此过渡问题,因为它位于以1625 cm –1为中心的宽带下,这是淀粉样蛋白纤维的传统红外特征。该特征在2D红外光谱中很显着,因为2D线形更窄,并且随着跃迁偶极子强度而非线性缩放。透射电子显微镜测量表明,1610 cm –1谱带是淀粉样蛋白原纤维的阳性鉴定。溶解和分解预聚集的Aβ样品的十二烷基硫酸钠胶束消耗1625 cm –1谱带,但不影响1610 cm –1谱带,表明1610 cm –1谱带是由于非常稳定的原纤维。我们证明了1610厘米–1过渡态是由酰胺I模式产生的,它是通过突变掉唯一可能引起该过渡的侧链残基而产生的,我们通过模拟基于Aβ晶体结构的2D红外光谱,探索了该过渡态的潜在结构起源。以前不可能用红外线将稳定的Aβ原纤维与较不稳定的富含β-折叠的低聚物区分开来。该2D IR签名将对阿尔茨海默氏症对Aβ聚集,原纤维形成和毒性的研究有用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号