当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Beyond DirectedorthoMetalation: Ru-Catalyzed CAr–O Activation/Cross-Coupling Reaction by Amide Chelation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-08-01 , DOI: 10.1021/ja503819x Yigang Zhao 1 , Victor Snieckus 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-08-01 , DOI: 10.1021/ja503819x Yigang Zhao 1 , Victor Snieckus 1
Affiliation
|
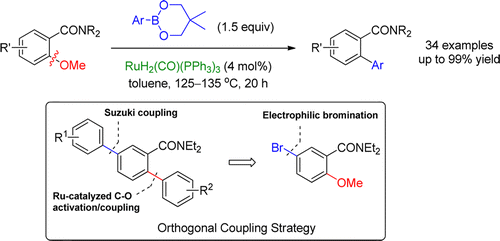
Disclosed is a new, catalytic, and general methodology for the chemical synthesis of biaryl, heterobiaryl, and polyaryl molecules by the cross-coupling of o-methoxybenzamides with aryl boroneopentylates. The reaction is based on the activation of the unreactive C-OMe bond by the proximate amide directing group using catalytic RuH2(CO)(PPh3)3 conditions. A one-step, base-free coupling process is thereby established that has the potential to supersede the useful two-step directed ortho metalation/cross-coupling reaction involving cryogenic temperature and strong base conditions. High regioselectivity, orthogonality with the Suzuki-Miyaura reaction, operational simplicity, minimum waste, and convenient scale-up make these reactions suitable for industrial applications.
中文翻译:

超越定向正金属化:Ru 催化的 CAr-O 活化/酰胺螯合的交叉偶联反应
公开了一种通过邻甲氧基苯甲酰胺与芳基硼戊酸酯的交叉偶联化学合成联芳基、杂联芳基和聚芳基分子的新的、催化的和通用的方法。该反应基于使用催化 RuH2(CO)(PPh3)3 条件通过邻近的酰胺导向基团激活非反应性 C-OMe 键。由此建立了一步、无碱偶联过程,它有可能取代有用的两步定向邻位金属化/交叉偶联反应,包括低温和强碱条件。高区域选择性、与 Suzuki-Miyaura 反应的正交性、操作简单、浪费最少和方便的放大使这些反应适合工业应用。
更新日期:2014-08-01
中文翻译:

超越定向正金属化:Ru 催化的 CAr-O 活化/酰胺螯合的交叉偶联反应
公开了一种通过邻甲氧基苯甲酰胺与芳基硼戊酸酯的交叉偶联化学合成联芳基、杂联芳基和聚芳基分子的新的、催化的和通用的方法。该反应基于使用催化 RuH2(CO)(PPh3)3 条件通过邻近的酰胺导向基团激活非反应性 C-OMe 键。由此建立了一步、无碱偶联过程,它有可能取代有用的两步定向邻位金属化/交叉偶联反应,包括低温和强碱条件。高区域选择性、与 Suzuki-Miyaura 反应的正交性、操作简单、浪费最少和方便的放大使这些反应适合工业应用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号