当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Na-Ion Solvation and High Transference Number in Superconcentrated Ionic Liquid Electrolytes: A Theoretical Approach
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.jpcc.7b09322 Fangfang Chen 1 , Patrick Howlett 1 , Maria Forsyth 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.jpcc.7b09322 Fangfang Chen 1 , Patrick Howlett 1 , Maria Forsyth 1
Affiliation
|
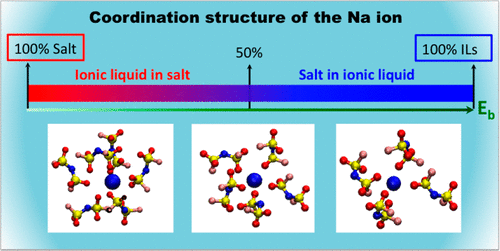
Ionic liquids have been extensively studied for developing next-generation Li- and Na-ion batteries because they are potentially safer replacements for conventional organic solvents in liquid electrolytes. Some recent work has drawn our attention to high lithium and sodium salt concentration ionic liquid systems which demonstrate high alkali-metal-ion transference numbers and support cycling at high current densities. Here we present an in-depth theoretical study of ion dynamics in a concentrated room-temperature ionic liquid, N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide ([C3mpyr][FSI]) with a sodium salt (NaFSI), focusing on how the solvation structure of a Na ion changes with salt concentration and how this affects its dynamics. These findings will help to understand the behavior of superconcentrated ionic liquid materials that will guide experimentalists in optimizing ionic liquid-based electrolyte materials.
中文翻译:

超浓缩离子液体电解质中的钠离子溶剂化和高转移数:一种理论方法
离子液体已被广泛研究用于开发下一代锂离子和钠离子电池,因为它们是液体电解质中常规有机溶剂的潜在安全替代品。最近的一些工作已引起我们对高锂和钠盐浓度离子液体系统的关注,该系统显示出高碱金属离子转移数并支持高电流密度下的循环。在这里,我们对浓缩的室温离子液体N-丙基-N-甲基吡咯烷鎓双(氟磺酰基)酰亚胺([C 3mpyr] [FSI])与钠盐(NaFSI),着重研究Na离子的溶剂化结构如何随盐浓度变化以及如何影响其动力学。这些发现将有助于理解超浓缩离子液体材料的行为,这将指导实验人员优化基于离子液体的电解质材料。
更新日期:2017-12-21
中文翻译:

超浓缩离子液体电解质中的钠离子溶剂化和高转移数:一种理论方法
离子液体已被广泛研究用于开发下一代锂离子和钠离子电池,因为它们是液体电解质中常规有机溶剂的潜在安全替代品。最近的一些工作已引起我们对高锂和钠盐浓度离子液体系统的关注,该系统显示出高碱金属离子转移数并支持高电流密度下的循环。在这里,我们对浓缩的室温离子液体N-丙基-N-甲基吡咯烷鎓双(氟磺酰基)酰亚胺([C 3mpyr] [FSI])与钠盐(NaFSI),着重研究Na离子的溶剂化结构如何随盐浓度变化以及如何影响其动力学。这些发现将有助于理解超浓缩离子液体材料的行为,这将指导实验人员优化基于离子液体的电解质材料。































 京公网安备 11010802027423号
京公网安备 11010802027423号