当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(III)-Catalyzed Cascade Annulations To Access Isoindolo[2,1-b]isoquinolin-5(7H)-ones via C–H Activation: Synthesis of Rosettacin
Organic Letters ( IF 4.9 ) Pub Date : 2017-12-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03509 Chada Raji Reddy 1, 2 , Kathe Mallesh 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2017-12-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03509 Chada Raji Reddy 1, 2 , Kathe Mallesh 1, 2
Affiliation
|
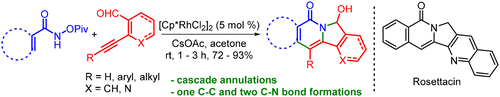
An efficient protocol for the synthesis of diversely substituted 7-hydroxyisoindolo[2,1-b]isoquinolin-5(7H)-ones from the reaction of N-(pivaloyloxy)benzamides with 2-alkynyl aldehydes has been developed, which proceeds through sequential alkyne insertion followed by addition of the amide nitrogen on to the aldehyde. This method provided the products with aminal functionality as a handle for further diversification. The synthetic utility of this strategy was successfully illustrated by the concise, two-step synthesis of an alkaloid, rosettacin, and a topoisomerase I inhibitor.
中文翻译:

Rh(III)催化级联环化通过C–H活化获得Iindindolo [2,1 - b ] isoquinolin -5(7 H)-ones:Rosettacin的合成
从N-(新戊酰氧基)苯甲酰胺与2-炔基醛的反应中合成了一个有效的协议,用于合成不同取代的7-羟基异吲哚并[2,1- b ]异喹啉-5(7 H)-,该过程通过依次进行炔烃插入,然后将酰胺氮加到醛上。这种方法为产品提供了具有动物功能的产品,以作为进一步多样化的手段。该策略的合成实用性通过生物碱,罗塞他星和拓扑异构酶I抑制剂的简明,两步合成得以成功说明。
更新日期:2017-12-07
中文翻译:

Rh(III)催化级联环化通过C–H活化获得Iindindolo [2,1 - b ] isoquinolin -5(7 H)-ones:Rosettacin的合成
从N-(新戊酰氧基)苯甲酰胺与2-炔基醛的反应中合成了一个有效的协议,用于合成不同取代的7-羟基异吲哚并[2,1- b ]异喹啉-5(7 H)-,该过程通过依次进行炔烃插入,然后将酰胺氮加到醛上。这种方法为产品提供了具有动物功能的产品,以作为进一步多样化的手段。该策略的合成实用性通过生物碱,罗塞他星和拓扑异构酶I抑制剂的简明,两步合成得以成功说明。































 京公网安备 11010802027423号
京公网安备 11010802027423号