当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diversity-oriented Synthesis of Inner Core Oligosaccharides of the Lipopolysaccharide of Pathogenic Gram-negative Bacteria
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-04-10 , DOI: 10.1021/ja401164s You Yang 1, 2 , Shunsuke Oishi 1, 2 , Christopher E. Martin 1, 2 , Peter H. Seeberger 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-04-10 , DOI: 10.1021/ja401164s You Yang 1, 2 , Shunsuke Oishi 1, 2 , Christopher E. Martin 1, 2 , Peter H. Seeberger 1, 2
Affiliation
|
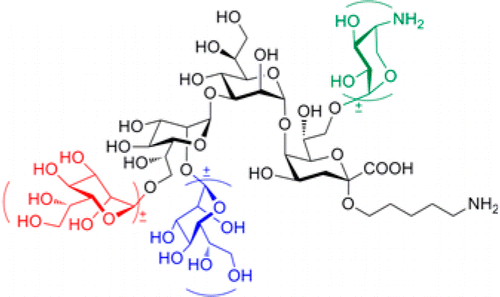
Lipopolysaccharide (LPS) is a potent virulence factor of pathogenic Gram-negative bacteria. To better understand the role of LPS in host-pathogen interactions and to elucidate the antigenic and immunogenic properties of LPS inner core region, a collection of well-defined L-glycero-D-manno-heptose (Hep) and 3-deoxy-α-D-manno-oct-2-ulosonic acid (Kdo)-containing inner core oligosaccharides is required. To address this need, we developed a diversity-oriented approach based on a common orthogonal protected disaccharide Hep-Kdo. Utilizing this new approach, we synthesized a range of LPS inner core oligosaccharides from a variety of pathogenic bacteria including Y. pestis, H. influenzae, and Proteus that cause plague, meningitis, and severe wound infections, respectively. Rapid access to these highly branched core oligosaccharides relied on elaboration of the disaccharide Hep-Kdo core as basis for the elongation with various flexible modules including unique Hep and 4-amino-4-deoxy-β-L-arabinose (Ara4N) monosaccharides and branched Hep-Hep disaccharides. A regio- and stereoselective glycosylation of Kdo 7,8-diol was key to selective installation of the Ara4N moiety at the 8-hydroxyl group of Kdo moiety of the Hep-Kdo disaccharide. The structure of the LPS inner core oligosaccharides was confirmed by comparison of (1)H NMR spectra of synthetic antigens and isolated fragments. These synthetic LPS core oligosaccharides can be covalently bound to carrier proteins via the reducing end pentyl amine linker, to explore their antigenic and immunogenic properties as well as potential applications such as diagnostic tools and vaccines.
中文翻译:

革兰氏阴性菌脂多糖内核寡糖的多样性导向合成
脂多糖 (LPS) 是致病性革兰氏阴性菌的强毒力因子。为了更好地了解 LPS 在宿主 - 病原体相互作用中的作用并阐明 LPS 内核区域的抗原和免疫原性特性,一组明确定义的 L-甘油-D-甘露糖-庚糖 (Hep) 和 3-脱氧-α需要含有-D-甘露糖-oct-2-ulosonic 酸 (Kdo) 的内核寡糖。为了满足这一需求,我们开发了一种基于常见正交保护二糖 Hep-Kdo 的面向多样性的方法。利用这种新方法,我们从包括鼠疫杆菌、流感嗜血杆菌和变形杆菌在内的多种致病细菌中合成了一系列 LPS 内核寡糖,这些细菌分别引起鼠疫、脑膜炎和严重伤口感染。快速获得这些高度支化的核心寡糖依赖于双糖 Hep-Kdo 核心的精心设计,作为延伸各种灵活模块的基础,包括独特的 Hep 和 4-氨基-4-脱氧-β-L-阿拉伯糖 (Ara4N) 单糖和支链Hep-Hep 二糖。Kdo 7,8-二醇的区域选择性和立体选择性糖基化是在 Hep-Kdo 二糖的 Kdo 部分的 8-羟基上选择性安装 Ara4N 部分的关键。通过比较合成抗原和分离片段的 (1) H NMR 光谱,证实了 LPS 内核寡糖的结构。这些合成的 LPS 核心寡糖可以通过还原末端戊胺接头与载体蛋白共价结合,
更新日期:2013-04-10
中文翻译:

革兰氏阴性菌脂多糖内核寡糖的多样性导向合成
脂多糖 (LPS) 是致病性革兰氏阴性菌的强毒力因子。为了更好地了解 LPS 在宿主 - 病原体相互作用中的作用并阐明 LPS 内核区域的抗原和免疫原性特性,一组明确定义的 L-甘油-D-甘露糖-庚糖 (Hep) 和 3-脱氧-α需要含有-D-甘露糖-oct-2-ulosonic 酸 (Kdo) 的内核寡糖。为了满足这一需求,我们开发了一种基于常见正交保护二糖 Hep-Kdo 的面向多样性的方法。利用这种新方法,我们从包括鼠疫杆菌、流感嗜血杆菌和变形杆菌在内的多种致病细菌中合成了一系列 LPS 内核寡糖,这些细菌分别引起鼠疫、脑膜炎和严重伤口感染。快速获得这些高度支化的核心寡糖依赖于双糖 Hep-Kdo 核心的精心设计,作为延伸各种灵活模块的基础,包括独特的 Hep 和 4-氨基-4-脱氧-β-L-阿拉伯糖 (Ara4N) 单糖和支链Hep-Hep 二糖。Kdo 7,8-二醇的区域选择性和立体选择性糖基化是在 Hep-Kdo 二糖的 Kdo 部分的 8-羟基上选择性安装 Ara4N 部分的关键。通过比较合成抗原和分离片段的 (1) H NMR 光谱,证实了 LPS 内核寡糖的结构。这些合成的 LPS 核心寡糖可以通过还原末端戊胺接头与载体蛋白共价结合,




















































 京公网安备 11010802027423号
京公网安备 11010802027423号