当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the native Sec61 protein-conducting channel.
Nature Communications ( IF 14.7 ) Pub Date : 2015-Sep-28 , DOI: 10.1038/ncomms9403
Stefan Pfeffer , Laura Burbaum , Pia Unverdorben , Markus Pech , Yuxiang Chen , Richard Zimmermann , Roland Beckmann , Friedrich Förster
Nature Communications ( IF 14.7 ) Pub Date : 2015-Sep-28 , DOI: 10.1038/ncomms9403
Stefan Pfeffer , Laura Burbaum , Pia Unverdorben , Markus Pech , Yuxiang Chen , Richard Zimmermann , Roland Beckmann , Friedrich Förster
|
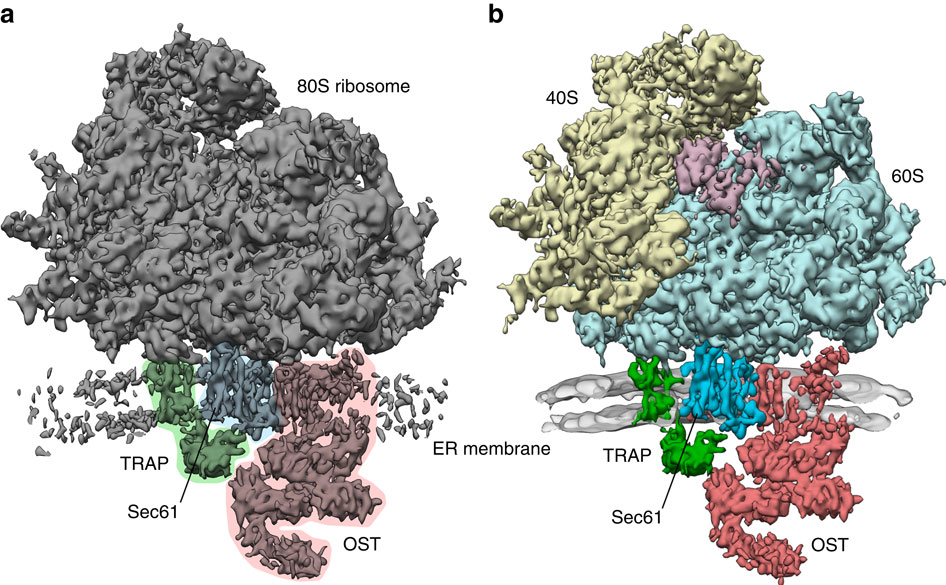
In mammalian cells, secretory and membrane proteins are translocated across or inserted into the endoplasmic reticulum (ER) membrane by the universally conserved protein-conducting channel Sec61, which has been structurally studied in isolated, detergent-solubilized states. Here we structurally and functionally characterize native, non-solubilized ribosome-Sec61 complexes on rough ER vesicles using cryo-electron tomography and ribosome profiling. Surprisingly, the 9-Å resolution subtomogram average reveals Sec61 in a laterally open conformation, even though the channel is not in the process of inserting membrane proteins into the lipid bilayer. In contrast to recent mechanistic models for polypeptide translocation and insertion, our results indicate that the laterally open conformation of Sec61 is the only conformation present in the ribosome-bound translocon complex, independent of its functional state. Consistent with earlier functional studies, our structure suggests that the ribosome alone, even without a nascent chain, is sufficient for lateral opening of Sec61 in a lipid environment.
中文翻译:

天然Sec61蛋白传导通道的结构。
在哺乳动物细胞中,分泌蛋白和膜蛋白通过普遍保守的蛋白传导通道Sec61跨内质网(ER)膜转移或插入内质网(ER)膜,该结构已在分离的去污剂溶解状态下进行了结构研究。在这里,我们在结构和功能上使用低温电子层析成像和核糖体谱分析对粗糙的ER囊泡上的天然,未溶解的核糖体Sec61复合物进行结构表征。出乎意料的是,即使通道不在将膜蛋白插入脂质双层中的过程中,9-Å分辨率的子层析图平均值也显示了Sec61呈侧向开放构型。与最新的多肽易位和插入机制模型不同,我们的结果表明,Sec61的侧向开放构象是存在于核糖体结合的translocon复合物中的唯一构象,而与它的功能状态无关。与早期的功能研究一致,我们的结构表明,即使没有新生链,单独的核糖体也足以在脂质环境中横向打开Sec61。
更新日期:2015-10-01
中文翻译:

天然Sec61蛋白传导通道的结构。
在哺乳动物细胞中,分泌蛋白和膜蛋白通过普遍保守的蛋白传导通道Sec61跨内质网(ER)膜转移或插入内质网(ER)膜,该结构已在分离的去污剂溶解状态下进行了结构研究。在这里,我们在结构和功能上使用低温电子层析成像和核糖体谱分析对粗糙的ER囊泡上的天然,未溶解的核糖体Sec61复合物进行结构表征。出乎意料的是,即使通道不在将膜蛋白插入脂质双层中的过程中,9-Å分辨率的子层析图平均值也显示了Sec61呈侧向开放构型。与最新的多肽易位和插入机制模型不同,我们的结果表明,Sec61的侧向开放构象是存在于核糖体结合的translocon复合物中的唯一构象,而与它的功能状态无关。与早期的功能研究一致,我们的结构表明,即使没有新生链,单独的核糖体也足以在脂质环境中横向打开Sec61。

































 京公网安备 11010802027423号
京公网安备 11010802027423号